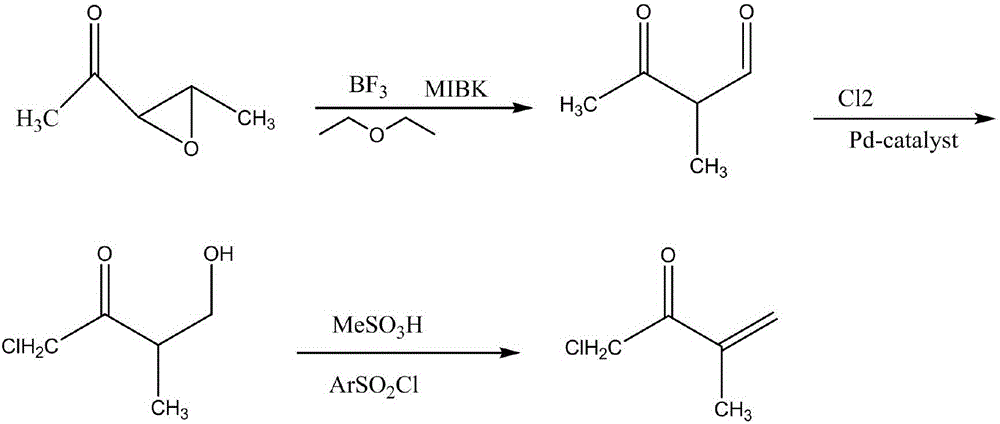
Synthetic method for 1-chloro-3-methyl-3-buten-2-one
A synthesis method and technology of methyl isobutyl, applied in the preparation of carbon-based compounds, chemical instruments and methods, preparation of heterocyclic compounds, etc., can solve the problems of difficult to scale up production, danger, and long synthesis route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0017] Add 5mL of 1-(3-methyloxiranyl) ethyl ketone, 10mL of ether and 2mL of methyl isobutyl (methyl) ketone into a 50ml round bottom flask, mix well, and then use boron fluoride to dissolve the The air is exhausted, the flask is sealed, and after shaking by hand for 2 minutes, the gas in the flask is collected, and then 2 zeolites are added, and then a condenser tube is installed; the above-mentioned round bottom flask is heated and kept under reflux for 1 hour, then the heating is stopped, and the After the reactant was cooled to room temperature, the reflux device was changed to a distillation device, the receiving bottle was cooled with cold water, and heated to 114°C until the volume of the distillate was about 40% of the total volume of the reactant, and the effluent was collected to obtain 3 -Methyl-2,4-butanedione; add 10 mL of distilled water to the distillate obtained above using a dropping funnel, drop it within 3 minutes, and keep shaking, then transfer the mixture...
example 2
[0019] Add 10mL of 1-(3-methyloxiranyl) ethanone, 15mL of ether and 6mL of methyl isobutyl (methyl) ketone into a 50ml round bottom flask, mix well, and then use boron fluoride to dissolve the Vent the air, seal the flask, shake it by hand for 3 minutes, collect the gas in the flask, then add 2 zeolites, and then install a condenser tube; heat the above-mentioned round bottom flask, and keep it under reflux for 2 hours, then stop heating, wait for the After the reactant was cooled to room temperature, the reflux device was changed into a distillation device, the receiving bottle was cooled with cold water, and heated to 126°C until the volume of the distillate was about 60% of the total volume of the reactant, and the effluent was collected to obtain 3 -Methyl-2,4-butanedione; add 10 mL of distilled water to the distillate obtained above using a dropping funnel, drop it within 5 minutes, and keep shaking, then transfer the mixture into a beaker Pour 20mL of chlorine into it, s...
example 3
[0021] In a 50ml round bottom flask, add 10mL1-(3-methyloxiranyl)ethanone, 10mL ether and 4mL methylisobutyl (methyl)ketone, mix well, and then use boron fluoride to dissolve the The air is discharged, the flask is sealed, and after shaking by hand for 3 minutes, the gas in the flask is collected, and then 2 zeolites are added, and then a condenser is installed; the above-mentioned round bottom flask is heated and kept under reflux for 1 hour, then the heating is stopped, and the After the reactant was cooled to room temperature, the reflux device was changed to a distillation device, the receiving bottle was cooled with cold water, and heated to 118°C until the volume of the distillate was about 50% of the total volume of the reactant, and the effluent was collected to obtain 3 -Methyl-2,4-butanedione; add 10 mL of distilled water to the distillate obtained above using a dropping funnel, drop it within 5 minutes, and keep shaking, then transfer the mixture into a beaker Pour ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com