Benzoxazolyl isoquinoline cuprous complex orange red phosphorescent material in crystal form
A technology of benzoxazolyl and phosphorescent materials, which is applied in the field of luminescent materials and organic electroluminescent materials, can solve the problems that the luminous intensity cannot meet the application requirements, achieve good phosphorescent emission performance, promote inter-system crossing, and process easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A large number of phosphorescent complex materials [Cu(2-iQBO)(p-Tol 3 P) 2 ]PF 6 Preparation of crystal sample: weigh 0.037g (0.1mmol) of Cu(CH 3 CN) 4 PF 6 , 0.061g (0.2mmol) of p-Tol 3 P, 0.024g (0.1mmol) of 2-iQBO; respectively dissolve in 3ml of dichloromethane and mix in sequence, stir well to make the coordination reaction fully occur, and obtain an orange-red clear solution; add a small amount of isopropanol to the above solution , And rotary evaporation at room temperature to remove all solvents, and finally an orange crystal product was obtained with a yield of 91% (calculated by Cu).
Embodiment 2
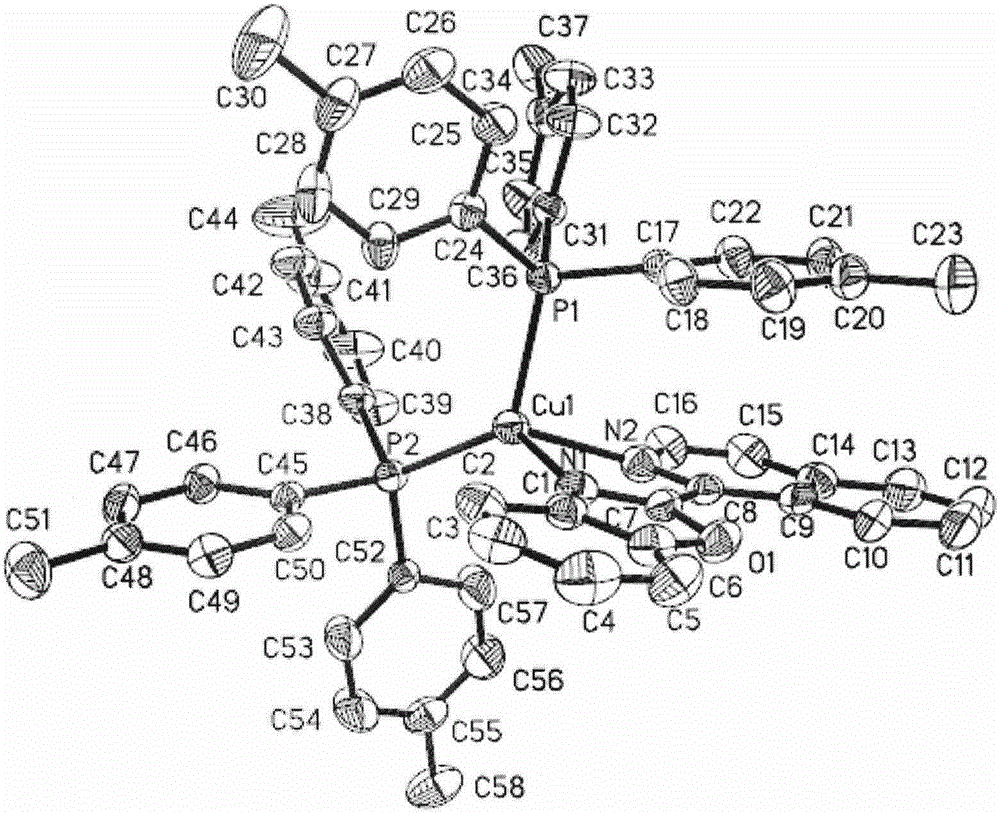
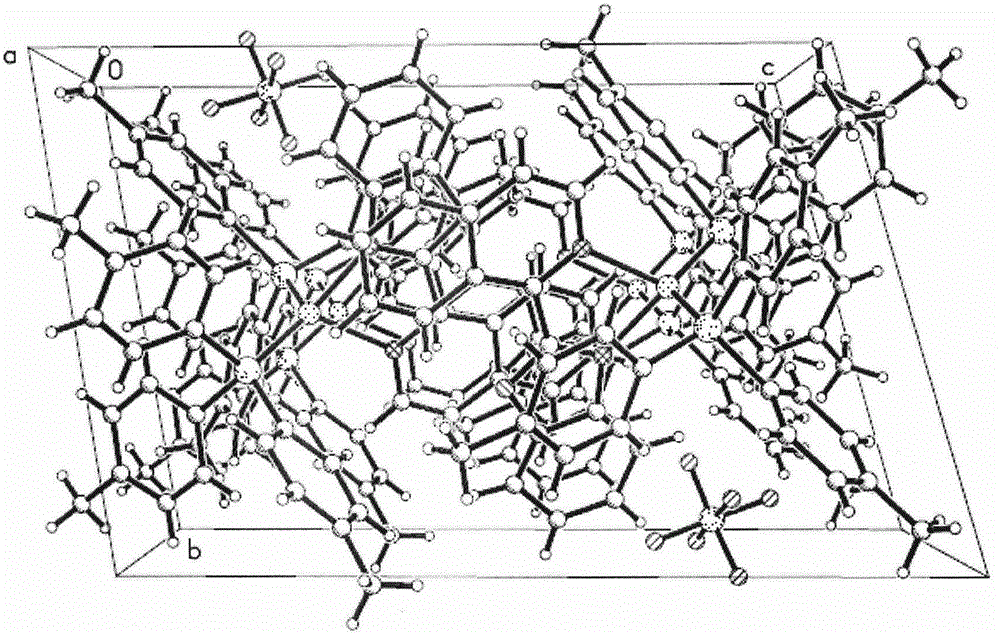
[0031] Synthesis of orange-red phosphorescent complex material [Cu(2-iQBO)(p-Tol 3 P) 2 ]PF 6 Single crystal: weigh 0.037g (0.1mmol) of Cu(CH 3 CN) 4 PF 6 , 0.061g (0.2mmol) of p-Tol 3 P, 0.024g (0.1mmol) of 2-iQBO; respectively dissolve in 4ml of dichloromethane and mix in sequence, stir well to make the coordination reaction fully occur, and obtain orange-red clear solution; after concentration under reduced pressure at room temperature, The upper layer was covered with isopropanol to promote the crystallization of the product, and a large amount of orange lumpy crystals precipitated after a few days of standing. Choose a square orange crystal with a size of 0.42mm*0.40mm*0.36mm for X-ray single crystal structure testing. The molecular structure diagram of the compound is attached figure 1 , Its unit cell stacked structure diagram is attached figure 2 .
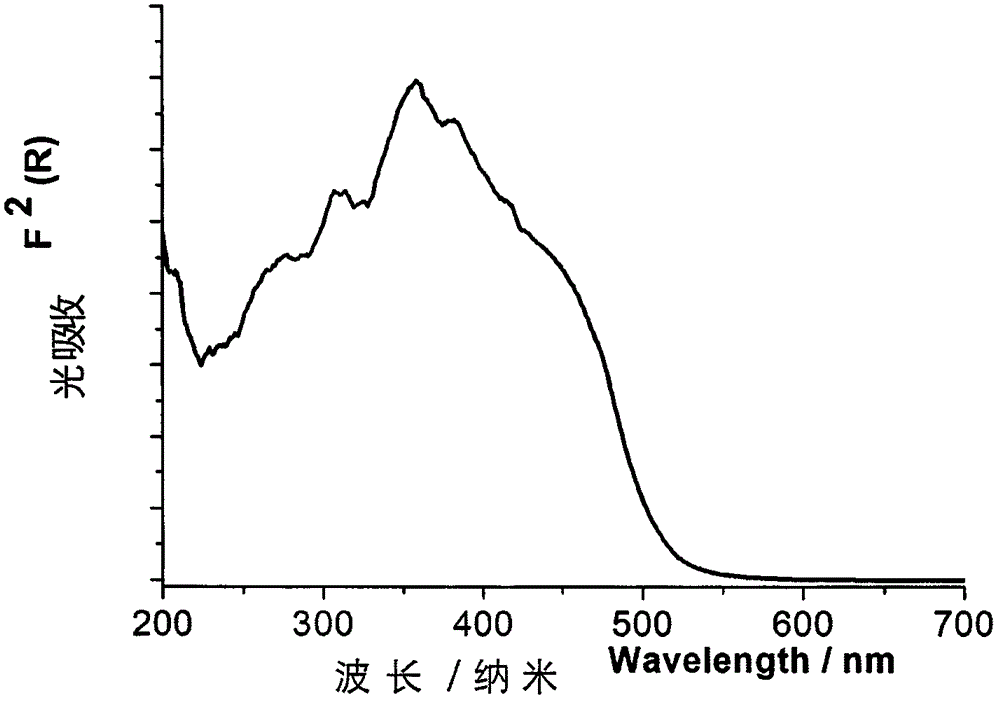
[0032] For orange-red phosphorescent complex material [Cu(2-iQBO)(p-Tol 3 P) 2 ]PF 6 A series of performance tests were car...
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence lifetime | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com