Medicine composition for treating diabetic neuropathy and preparing method thereof
A technology of composition and medicine, applied in the field of epalrestat pharmaceutical composition and its preparation, can solve the problems of low average dissolution rate of epalrestat tablets, large difference in dissolution rate between tablets, etc., and achieve the reduction of dissolution rate difference Small size, improved average dissolution rate, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
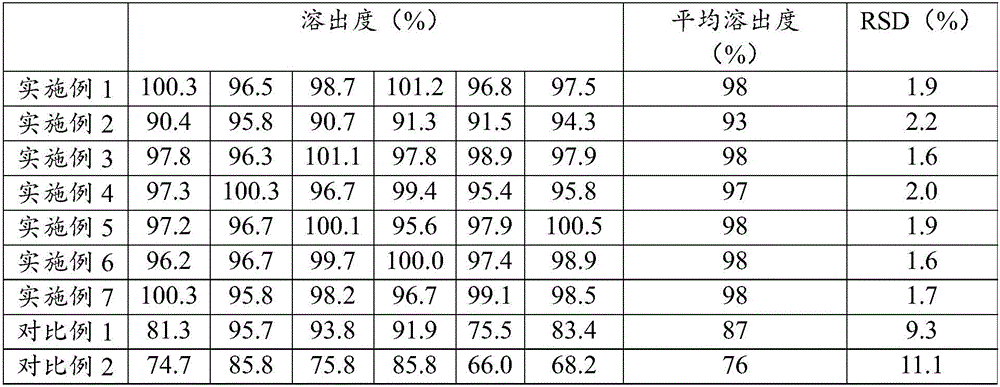
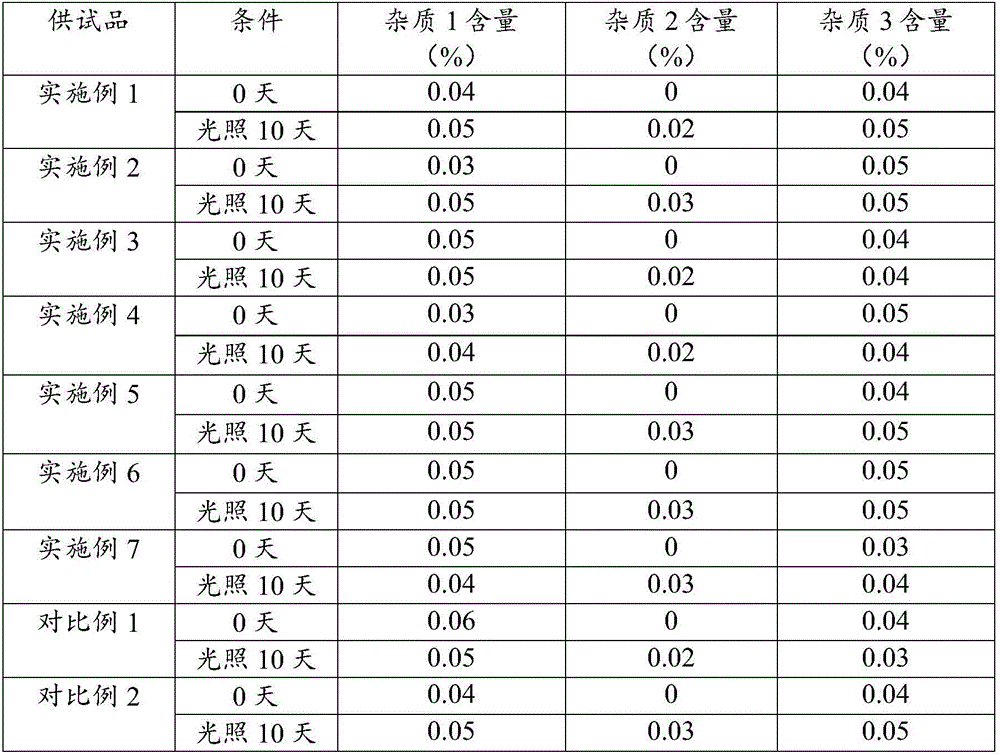
Examples
Embodiment 1
[0061] The present embodiment epalrestat tablet, its raw material consists of:
[0062] Epalrestat 50mg, polyethylene glycol 4000 50mg, mannitol 80mg, hydroxypropyl cellulose 10mg, croscarmellose calcium 10mg, magnesium stearate 3mg, appropriate amount of water, appropriate amount of 50% ethanol aqueous solution, hydroxypropyl Methylcellulose 16.2mg, titanium dioxide 9.5mg, polyethylene glycol 1.3mg;
[0063] Its preparation method comprises the following steps:
[0064] Take the raw and auxiliary materials of the prescription amount respectively, pulverize and sieve respectively, and the particle diameter (D90) of epalrestat is 15 μm;
[0065] Preparing the extrudate by hot-melt extrusion method at 55-70°C with the prescribed amount of epalrestat and polyethylene glycol 4000, cooled at room temperature, pulverized, and sieved to obtain mixed powder;
[0066] Mix the mixed powder with the prescribed amount of filler evenly, use the prescribed amount of hydroxypropyl cellulos...
Embodiment 2
[0070] The present embodiment epalrestat tablet, its raw material consists of:
[0071] The composition of the raw and auxiliary materials of the epalrestat tablet of this embodiment is identical with the composition of the raw and auxiliary materials of the epalrestat tablet of embodiment 1;
[0072] The difference between the raw and auxiliary material composition of the epalrestat tablet in this embodiment and the preparation method of the epalrestat tablet in Example 1 is only that the particle diameter (D90) of epalrestat is 110 μm, and all the other experimental conditions and operating steps Same as embodiment 1;
Embodiment 3
[0074] The present embodiment epalrestat tablet, its raw material consists of:
[0075] Epalrestat 50mg, polyethylene glycol 4000 55mg, titanium dioxide 4.4mg, mannitol 80mg, hydroxypropyl cellulose 10mg, cross-linked carboxymethyl calcium 10mg, magnesium stearate 3mg, appropriate amount of water, appropriate amount of 50% ethanol aqueous solution , hydroxypropyl methylcellulose 2.5mg, polyethylene glycol 0.2mg;
[0076] Its preparation method comprises the following steps:
[0077] Take the raw and auxiliary materials of the prescription amount respectively, pulverize and sieve respectively, and the particle diameter (D90) of epalrestat is 15 μm;
[0078] Prepare the extrudate by hot-melt extrusion from the prescribed amount of epalrestat, 3 mg of titanium dioxide and polyethylene glycol 4000 at 55-70°C, cool at room temperature, pulverize, and sieve to obtain a mixed powder;
[0079] Mix the mixed powder with the prescribed amount of filler evenly, use the prescribed amoun...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com