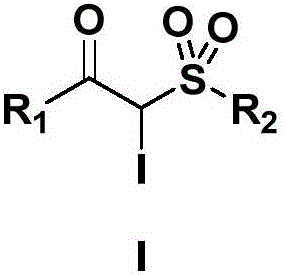
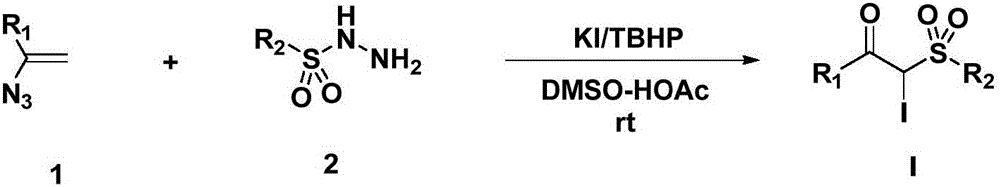
Preparation method for alpha-iodo-beta-arylketo-substituted sulfone compounds
A compound and aryl ketone-based technology, applied in the field of compound synthesis, can solve problems such as harsh reaction conditions, low reaction efficiency, and difficult to obtain raw materials, and achieve the effect of simple operation, easy-to-obtain raw materials, and cheap and easy-to-obtain reaction raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
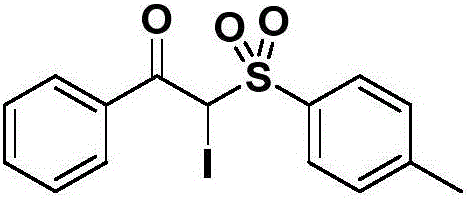
[0028] Example 1: Preparation of 2-iodo-1-phenyl-2-(4-methyl)benzenesulfonyl-1-one
[0029] Add 290 mg (2 mmol) of 1-azidenylbenzene, 744 mg (4 mmol, 2.0 eq.) of p-methylbenzenesulfonyl hydrazide and 664 mg (4 mmol, 2.0 eq.) of potassium iodide into the reaction flask, and then add 1 mL of tert-butyl peroxide Alcohol and 4 mL DMSO-HOAc (V; V=1:1) were reacted at room temperature for 4 hours. After the TLC plate detects that the raw materials disappear, add water to dilute the system, extract with ethyl acetate, dry the organic phase, evaporate the solvent under reduced pressure, and purify the crude product by column chromatography (eluent: petroleum ether: ethyl acetate = 1:2) A white solid was obtained with a yield of 85%, melting point: 168-169°C,
[0030] Its structural formula is:
[0031]
[0032] 1 H NMR (500MHz, CDCl 3 )δ7.90(dd, J=12.5,8.0Hz,4H),7.63(t,J=7.5Hz,1H),7.48(t,J=8.0Hz,2H),7.35(d,J=8.0Hz, 2H), 6.52(s, 1H), 2.45(s, 3H). 13 C NMR (125MHz, CDCl 3 )δ18...
Embodiment 2
[0033] Example 2: Preparation of 2-iodo-1-(3-bromo-4-ethoxy)phenyl-2-(4-methyl)benzenesulfonyl-1-one
[0034] The synthesis method is the same as in Example 1, except that the raw material 1-azidenylbenzene is replaced with 1-azidonyl-(3-bromo-4-ethoxyl)benzene. Off-white solid, yield 80%, melting point: 172-173°C, its structural formula is:
[0035]
[0036] 1 H NMR (500MHz, CDCl 3 )δ8.08(d, J=2.0Hz, 1H), 7.90(dd, J=8.5, 2.5Hz, 1H), 7.86(d, J=8.0Hz, 2H), 7.35(d, J=8.0Hz, 2H), 6.90(d, J=8.5Hz, 1H), 6.42(s, 1H), 4.19(q, J=7.0Hz, 2H), 2.45(s, 3H), 1.51(t, J=7.0Hz, 3H). 13 C NMR (125MHz, CDCl 3 )δ185.7, 160.4, 146.3, 134.8, 132.2, 131.0, 130.9, 129.7, 128.7, 127.1, 112.9, 112.1, 65.5, 38.2, 21.9, 14.6. HRMS (ESI): m / zcalcd for (C 17 h 16 BrIO 4 S+H) + :522.9070; found: 522.9074.
Embodiment 3
[0037] Example 3: Preparation of 2-iodo-1-(4-bromo)phenyl-2-(4-methyl)benzenesulfonyl-1-one
[0038] The synthesis method is the same as in Example 1, except that the raw material 1-azidenylbenzene is replaced with 1-azidoenyl-4-bromobenzene. Off-white solid, yield 86%, melting point: 147-148°C, its structural formula is:
[0039]
[0040] 1 H NMR (500MHz, DMSO) δ7.89-7.88(m, 2H), 7.83(d, J=8.0Hz, 2H), 7.73-7.71(m, 2H), 7.45(s, 1H), 7.41(d, J=8.0Hz,2H),2.38(s,2H). 13 C NMR (125MHz, DMSO) δ189.0, 145.2, 133.1, 132.5, 132.0, 130.8, 129.6, 129.5, 128.8, 39.2, 21.2. HRMS (ESI): m / z calcd for (C 15 h 12 BrIO 3 S+H) + :478.8808; found: 478.8809.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com