Sensor molecules for identifying cyanide ion and hydrogen sulfate ion in relayed manner, synthesis and application thereof
A hydrogen sulfate ion, sensor technology, applied in the field of anion detection, chemical synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1. Synthesis and characterization of sensor molecule T
[0059] Put 2 mmol of 2,3-diaminophenazine and 2.5 mmol of benzaldehyde in a 100 mL round bottom flask, add 20 mL of DMF, add 0.1 mL of glacial acetic acid, heat and reflux on an oil bath at 85°C for 8 hours, and cool to room temperature after the reaction is complete , after neutralization, suction filtration; the obtained solid was washed 3 to 5 times with hot ethanol, dried in a vacuum oven, and then washed with DMF-H 2 O was recrystallized, and the obtained brown solid was the sensor molecule T, with a yield of 75%.
[0060] T: m.p. > 300 C; 1H NMR (DMSO-d6, 400 MHz) d 13.45 (1H, NH), d 12.95 (1H, OH), d 8.43-8.24 (2H, ArH) 7.91-7.89 (2H, ArH) 7.56-7.53 (1H, ArH) 7.17-7.11 (2H, ArH). 13 C NMR (DMSO-d6, 150 MHz) d 172.52, 160.23, 141.83, 140.44, 129.47, 129.28, 128.55, 119.32, 117.75. IR (KBr, cm1) v: 3310.70 (O-H), 3047.32 (N-H) =N), 1613.04 (Ar, C=C), 1528.76 (Ar, C=C), 1488.12(Ar, C=C). ESI-MS m...
Embodiment 2
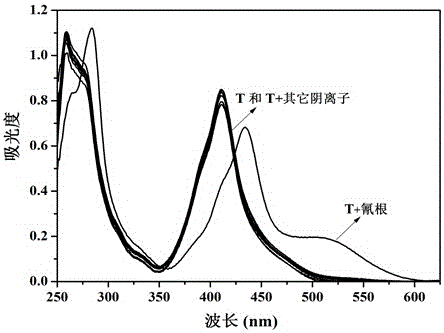
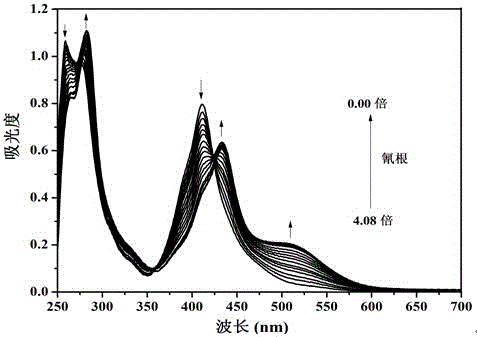
[0063] Embodiment 2. Colorimetric relay recognition of cyanide ion and hydrogen sulfate ion by sensor molecule T
[0064] Pipette 0.5 mL of sensor molecule T in DMSO solution (2×10 -4 mol L -1 ) into a series of 10 mL colorimetric tubes, add F - , Cl - , Br - , I - , AcO - , H 2 PO - 4 , HSO - 4 , ClO - 4 , CN - and SCN - aqueous solution (1×10 -2 mol L -1 ); if the color of the solution of the sensor molecule T changes from yellow to orange, it means that the addition of CN - , if the color of the solution of the sensor molecule T does not change, it means that the addition of CN is not - .
[0065] Pipette 0.5 mL of sensor molecule T in DMSO solution (2×10 -4 mol L -1 ) into a series of 10 mL colorimetric tubes, respectively add CN - aqueous solution (1×10 -2 mol L -1 ), the color of sensor molecule T solution changed from yellow to orange. Then add F respectively - , Cl - , Br - , I - , AcO - , H 2 PO - 4 , HSO - 4 , ClO - 4 and SCN - ...
Embodiment 3
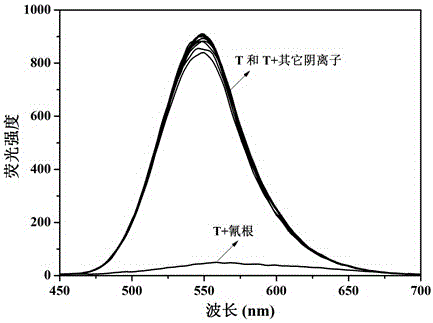
[0066] Example 3: Relay fluorescence recognition of cyanide ion and hydrogen sulfate ion by sensor molecule T
[0067] Pipette 0.5 mL of sensor molecule T in DMSO solution (2×10 -4 mol L -1 ) into a series of 10 mL colorimetric tubes, add F - , Cl - , Br - , I - , AcO - , H 2 PO - 4 , HSO - 4 , ClO - 4 , CN - and SCN - aqueous solution (1×10 -2 mol L -1 ); if the fluorescence of the solution of the sensor molecule T is completely quenched, it means that the addition of CN - , if the fluorescence of the solution of the sensor molecule T does not change, it means that the addition of CN is not - .
[0068] Pipette 0.5 mL of sensor molecule T in DMSO solution (2×10 -4 mol L -1 ) into a series of 10 mL colorimetric tubes, respectively add CN - aqueous solution (1×10 -2 mol L -1 ), the fluorescence of the solution of the sensor molecule T is completely quenched. Then add F respectively - , Cl - , Br - , I - , AcO - , H 2 PO - 4 , HSO - 4 , ClO -...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com