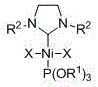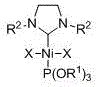Phosphite/N-heterocyclic carbene-containing mixed nickel (II) complex, and preparation method and application thereof
A nitrogen heterocyclic carbene and phosphite technology, applied in nickel complexes and in the field of organic synthesis, can solve problems such as no reports, no cross-coupling reaction of chlorinated hydrocarbons and neopentyl glycol biborates, etc. , to achieve the effect of easy product, conducive to large-scale synthesis and application, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment one: Ni[P(OR 1 ) 3 ][(R 2 NCH 2 CH 2 NR 2 )C]X2 (R 1 = CH 2 CH 3 , R 2 =2,4,6-trimethylphenyl, X = Br) synthesis
[0026] The azacyclic carbene (R 2 NCH 2 CH 2 NR 2 )C (0.2464g, 0.8 mmol) was added into a tetrahydrofuran solution of di(triethylphosphite)nickel(II) bromide (0.4400 g, 0.8 mmol), reacted at room temperature for 2 hours, and removed the solvent in vacuo, Wash the residue with n-hexane, extract the residue with toluene, transfer the clear liquid and remove the solvent toluene to obtain a red solid with a yield of 68%.
[0027] The product was subjected to elemental analysis, and the results are shown in Table 1:
[0028] Table 1 Elemental analysis results
[0029]
C:(%)
H:(%)
N:(%)
theoretical value
46.86
6.12
4.05
actual value
47.04
6.21
3.99
[0030] The product was characterized by NMR, and the results are as follows:
[0031] Dissolve the product in C 6 D. 6 Medium (abo...
Embodiment 2
[0032] Embodiment two: Ni[P(OR 1 ) 3 ][(R 2 NCH 2 CH 2 NR 2 )C]X 2 (R 1 = CH 2 CH 3 , R 2 = 2,6-Diisopropylphenyl, X = Br) Synthesis of
[0033] The azacyclic carbene (R 2 NCH 2 CH 2 NR 2 )C (0.3627 g, 0.93 mmol) was added into a tetrahydrofuran solution of two (triethyl phosphite) nickel (II) bromide (0.5115 g, 0.93 mmol), reacted at room temperature for 2 hours, and removed the solvent in vacuo, Wash the residue with n-hexane, extract the residue with toluene, transfer the clear liquid and remove the solvent toluene to obtain a red solid with a yield of 77%.
[0034] Product is carried out elemental analysis, and the result is as shown in table 2:
[0035] Table 2 Elemental Analysis
[0036]
C:(%)
H:(%)
N:(%)
theoretical value
51.06
7.01
3.61
actual value
51.33
7.19
3.49
[0037] The product was characterized by NMR, and the results are as follows:
[0038] Dissolve the product in C 6 D. 6 Medium (a...
Embodiment 3
[0039] Embodiment three: Ni[P(OR 1 ) 3 ][(R 2 NCH 2 CH 2 NR 2 )C]X 2 (R 1 = CH(CH 3 ) 2 , R 2 = 2,6-Diisopropylphenyl, X = Br) Synthesis of
[0040] The azacyclic carbene (R 2 NCH 2 CH 2 NR 2 )C (0.3627 g, 0.93 mmol) was added to a tetrahydrofuran solution of di(triisopropyl phosphite) nickel(II) bromide (0.5905 g, 0.93 mmol), reacted at room temperature for 3 hours, and removed the solvent in vacuo , wash the residue with n-hexane, extract the residue with toluene, transfer the clear liquid and remove the solvent toluene to obtain a red-black solid with a yield of 70%.
[0041] Product is carried out elemental analysis, and the result is as shown in table 3:
[0042] Table 3 Elemental analysis
[0043]
C:(%)
H:(%)
N:(%)
theoretical value
52.84
7.39
3.42
actual value
53.11
7.51
3.28
[0044] The product was characterized by NMR, and the results are as follows:
[0045] Dissolve the product in C 6 D. 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com