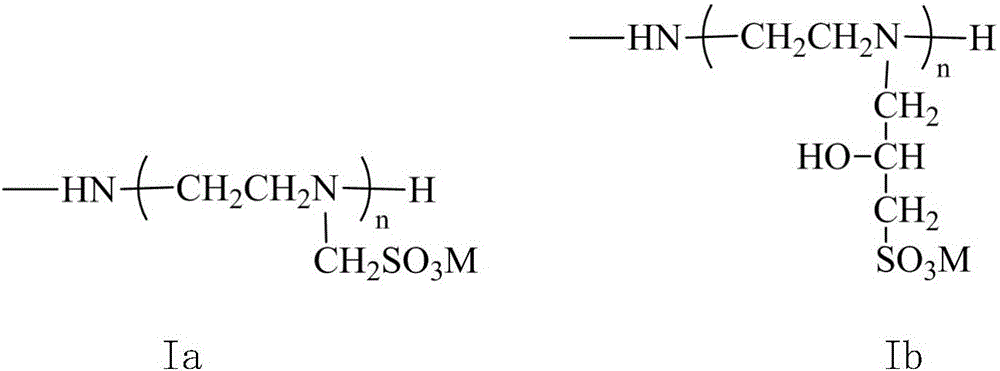High-solubility reactive dye and preparation method thereof
A technology of reactive dyes and high solubility, applied in the direction of reactive dyes, azo dyes, organic dyes, etc., can solve the problems of application limitations and low solubility, and achieve good solubility and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
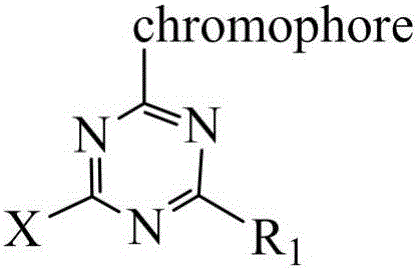
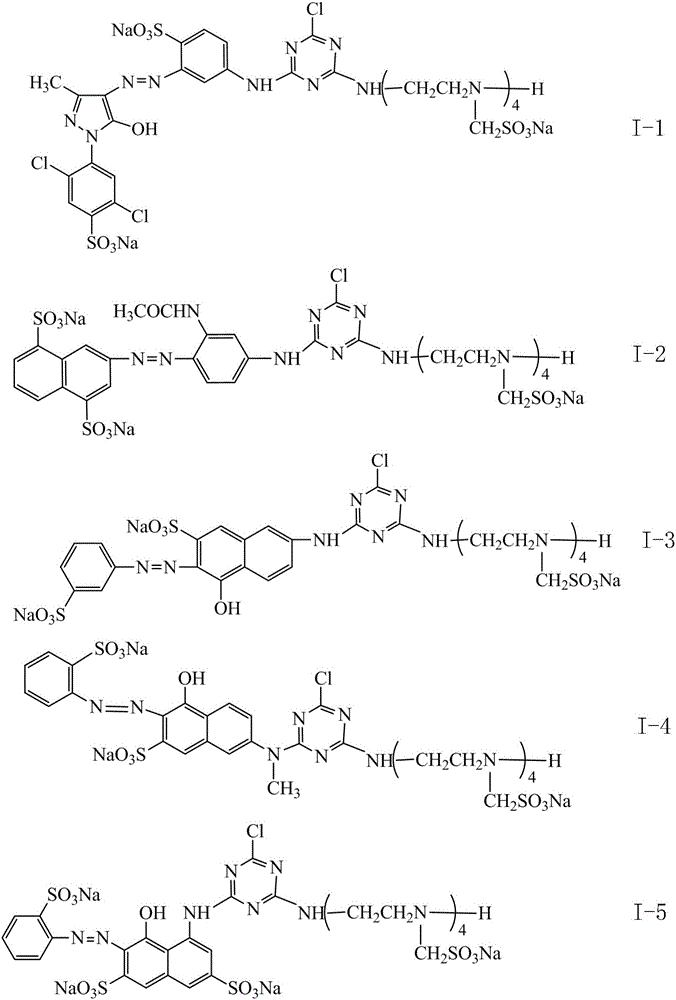
[0023] In a 500mL beaker, add 9.26g cyanuric chloride (0.0503mol), 50g ice and a small amount of water, beat at 0-5°C for 1 hour, and then add 100mL N-methyl J acid (12.65g, 0.05mol) solution ( Coupling components), the pH value was controlled at 5-6, the reaction was carried out for 2 hours, and the reaction end point was detected by Ehrlich reagent.
[0024] 3.47g of sodium nitrite was added to 70mL of anthranilic acid solution (8.65g, 0.05mol), stirred and dissolved, and then added to an aqueous hydrochloric acid solution cooled to below 10°C (12.5mL of concentrated hydrochloric acid was added to 15mL of water), reaction 1 hours, control the microscopic blue color of potassium iodide test paper, sulfamic acid eliminates excess nitrous acid, and obtains anthranilic acid diazonium salt solution. The above-mentioned anthranilic acid diazonium salt solution was added to the condensation product of cyanuric chloride and N-methyl J acid to carry out a coupling reaction, and the r...
Embodiment 2
[0027] The difference with the above-mentioned embodiment 1 is that in this example, the precursor of the corresponding high-solubility reactive dye is synthesized with ethylenediamine instead of tetraethylenepentamine, and then reacted with sodium hydroxymethanesulfonate to obtain a high-solubility reactive dyestuff. Dye, other conditions are the same as in Example 1.
Embodiment 3
[0029] The difference with the above-mentioned embodiment 1 is that in this example, the precursor of the corresponding highly soluble reactive dye is synthesized with diethylene triamine instead of tetraethylene pentamine, and then reacted with sodium hydroxymethanesulfonate to obtain a high solubility. Reactive dye, other conditions are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com