Positive electrode material for lithium-ion battery and preparation method of positive electrode material
A technology for lithium-ion batteries and positive electrode materials, applied in battery electrodes, secondary batteries, circuits, etc., can solve problems such as poor rate performance, fast cycle attenuation, Li/Ni mixed discharge, etc., to improve charge and discharge rate performance and Cycle performance, effect of improving electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
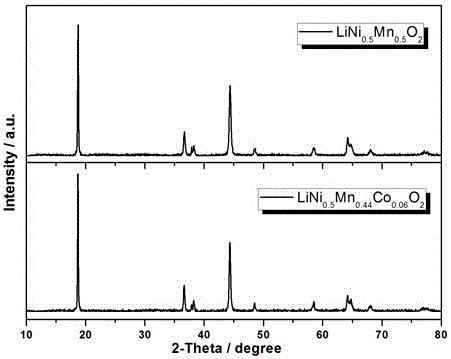
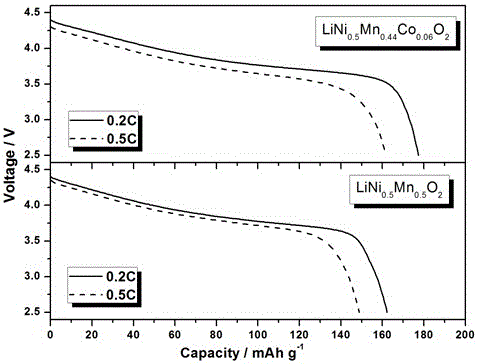
[0019] In this embodiment, when M=Co, x=0.06, and y=0, the chemical formula of the synthetic target product is LiNi 0.5 mn 0.44 co 0.06 o 2
[0020] (1) The hydroxide precursor material was prepared by co-precipitation method, and the molar ratio of Ni:Mn:Co was 0.5:0.44:0.06, and NiSO 4 ·6H 2 O, MnSO 4 ·H 2 O. CoSO 4 ·7H 2 Dissolve O in deionized water to prepare aqueous solution A with a cation concentration of 2mol / L; prepare 10mol / L ammonia water and 5mol / L NaOH solution at the same time; A is added to the reaction kettle at the same time, and 99.99% pure argon is introduced to protect it, and the pH is 11, and a precipitate is formed under stirring conditions; the solid obtained after the precipitate is washed and filtered is a hydroxide material of nickel manganese cobalt; the above precipitate Dry at 110°C to obtain solid powder;
[0021] (2) The molar ratio of Li:(Ni+Mn+Co) is 1.05:1, respectively weigh LiOH·H 2 O and the solid powder obtained in (1) are uni...
Embodiment 2
[0027]When M= Mg, x=0.04, y=0, the target product is LiNi 0.5 mn 0.46 Mg 0.04 o 2
[0028] (1) Preparation of hydroxide precursor materials by co-precipitation method: according to the cation molar ratio Ni:Mn:Mg is 0.5:0.46:0.04, respectively weigh NiSO 4 ·6H 2 O, MnSO 4 ·H 2 O, MgSO 4 ·7H 2 Dissolve O in deionized water to prepare aqueous solution A with a cation concentration of 1.5mol / L; prepare 5mol / L ammonia water and 5mol / L NaOH aqueous solution at the same time; A is added to the reaction kettle at the same time, and 99.99 wt.% purity nitrogen is introduced to protect it. At a pH of 10.8, a precipitate is formed under stirring conditions; the solid obtained after the precipitate is washed and filtered many times is a hydroxide material of nickel manganese magnesium; Calcining the above precipitate at 450°C to obtain a solid powder;
[0029] (2) According to the molar ratio Li:(Ni+Mn+Mg) is 1.02:1, weigh LiOH·H 2 O and the calcined product obtained in (1) are...
Embodiment 3
[0031] When M= Co, M′=Mg, x=0.02, y=0.02, the target product is LiNi 0.5 mn 0.46 co 0.02 Mg 0.02 o 2
[0032] (1) Preparation of hydroxide precursor materials by co-precipitation method: according to the metal cation molar ratio Ni:Mn:Co:Mg is 0.5:0.46:0.02:0.02, respectively weigh NiSO 4 ·6H 2 O, MnSO 4 ·H 2 O. CoSO 4 ·7H 2 O, MgSO 4 ·7H 2 Dissolve O in deionized water to prepare aqueous solution A with a cation concentration of 2.5mol / L; prepare 8mol / L ammonia water and 5mol / L NaOH aqueous solution at the same time; A is added to the reaction kettle at the same time, protected by argon with a purity of 99.99 wt.%, at a pH of 12, a precipitate is formed under stirring conditions, which is a hydroxide material of nickel manganese cobalt magnesium; the above precipitate is roasted at 800°C to obtain a metal oxide;
[0033] (2) The molar ratio of Li:(Ni+Mn+Co+Mg) is 1.02:1, respectively weigh LiOH·H 2 O and the calcined product obtained in (1) are uniformly mixed; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com