Carbon dioxide recycling method
A carbon dioxide, resource-recycling technology, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, preparation of organic compounds, etc., can solve problems such as large energy consumption, and achieve simple equipment and mild reaction conditions. , the effect of high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
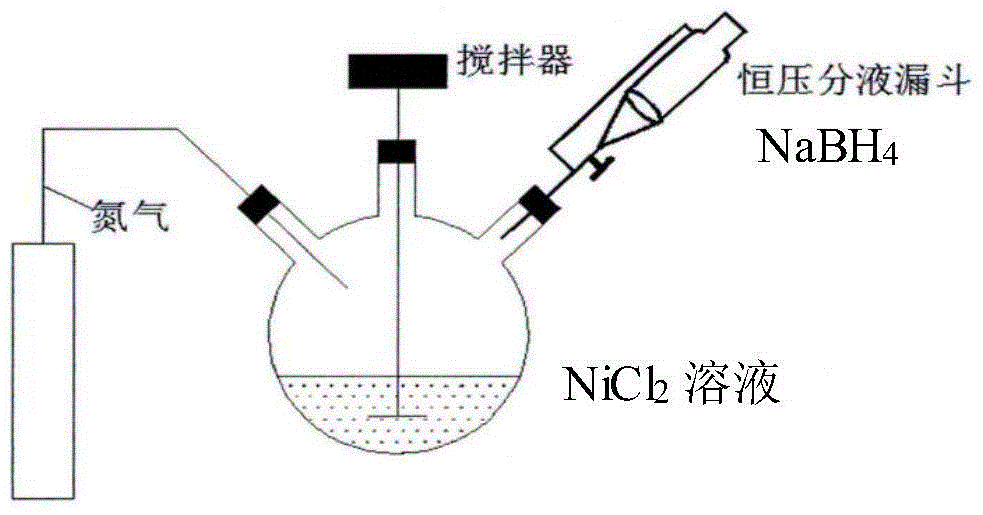
[0043] 38g NiCl 2 ·H 2 O was dissolved in a mixed solution of 500ml ethanol and water (the volume ratio of ethanol and water was 4:1), mixed thoroughly and transferred to a three-necked bottle; 14.16g NaBH 4 Add 1NaOH to 400ml anaerobic water as a reducing agent; expose N to the three-necked bottle 15 minutes in advance2 to remove NiCl 2 Oxygen in the solution, then while stirring vigorously, slowly add reducing agent to it through a constant pressure funnel. After the reducing agent is dripped, continue to stir vigorously for 30 minutes to ensure sufficient reaction, and then wash with anaerobic water and ethanol for 5~ 6 times, put it into a vacuum drying oven at 65° C. and dry overnight to obtain nano-nickel, which is stored in an anaerobic glove box for future use.
[0044] figure 1 Schematic diagram of the nano-nickel preparation device.
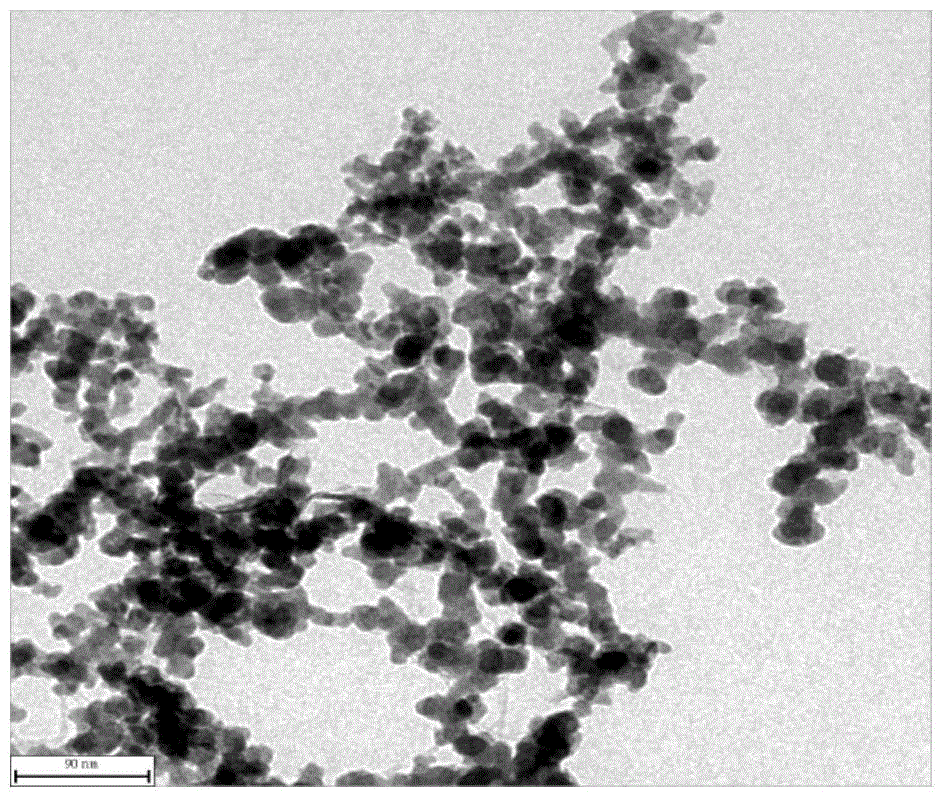
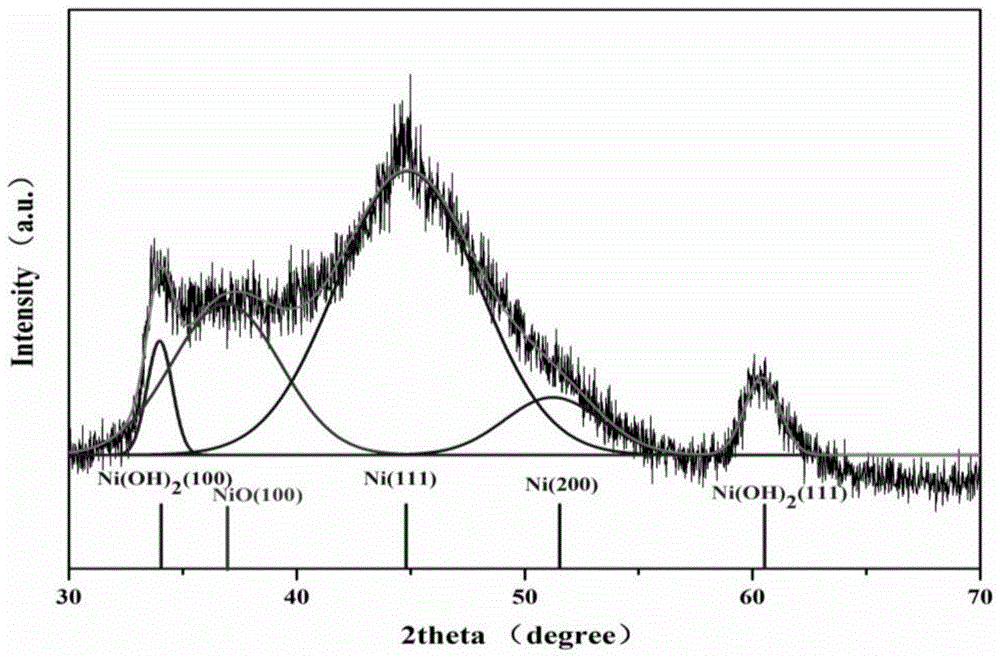
[0045] Utilize transmission electron microscope to analyze the nano-nickel obtained in embodiment 1, obtain its transmission elect...
Embodiment 2
[0048] 35ml 10mmol / L NaHCO 3 solution (as CO 2 Source) into a serum bottle with a total volume of 65ml, adjust the pH value to 6 with 5% dilute HCl by volume, and expose to N 2 10min to remove oxygen in the serum bottle, add 1g of nano-nickel prepared in Example 1 and expose to H 2 5min, make H 2 Fill the headspace of the serum bottle, cover with a rubber stopper, seal with an aluminum cap, and then expose a small amount of H 2 Make headspace final H 2 The pressure is 1.2 to 1.5 atmospheres, and the experiment has three parallel samples. Put the packed serum bottle into a shaker, control the temperature at 35°C, shake at a speed of 220rpm, and expose to H once every 24h. 2 Make the headspace pressure to 1 atmosphere, and regularly detect the headspace gas (GC) and liquid components (HPLC), such as Figure 4 as shown, Figure 4 is the HPLC chromatogram of the liquid components, given by Figure 4 It can be seen that the final product is determined to be formic acid. ...
Embodiment 3
[0051] 35ml 10mmol / L NaHCO 3 solution (as CO 2 Source) into a serum bottle with a total volume of 65ml, adjust the pH value to 6 with 5% dilute HCl by volume, and expose to N 2 10min to remove oxygen in the serum bottle, add 1g of nano-nickel prepared in Example 1 and expose to H 2 5min, make H 2 Fill the headspace of the serum bottle, cover with a rubber stopper, seal with an aluminum cap, and then expose a small amount of H 2 Make headspace final H 2 It is 1.2 to 1.5 atmospheres. Put the packed serum bottle into a shaker, control the temperature at 35°C, and shake at a speed of 220rpm. The experiment is divided into two groups, group A and group B. Group A is exposed to H once every 24 hours. 2 The headspace pressure was set at 1 atmosphere, and group B was only initially exposed to 1.2 to 1.5 atmospheres of H 2 No exposure to H during the post-reaction 2 , two groups of headspace gas (GC) and liquid components (HPLC) were regularly detected, and the standard relati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com