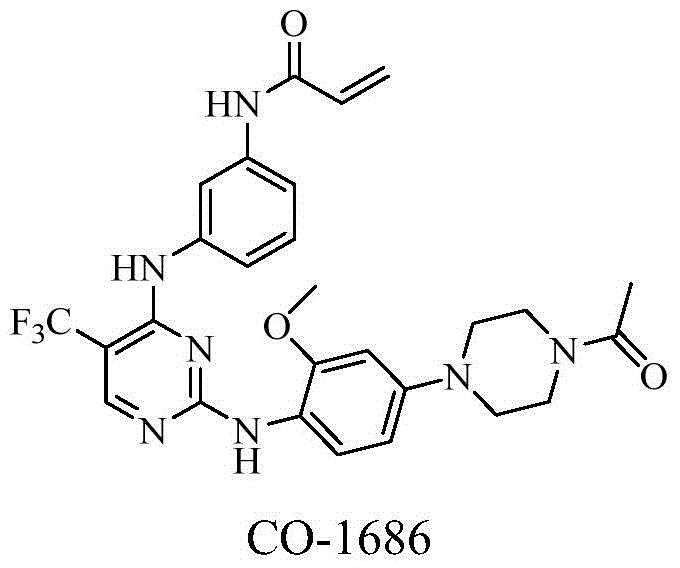
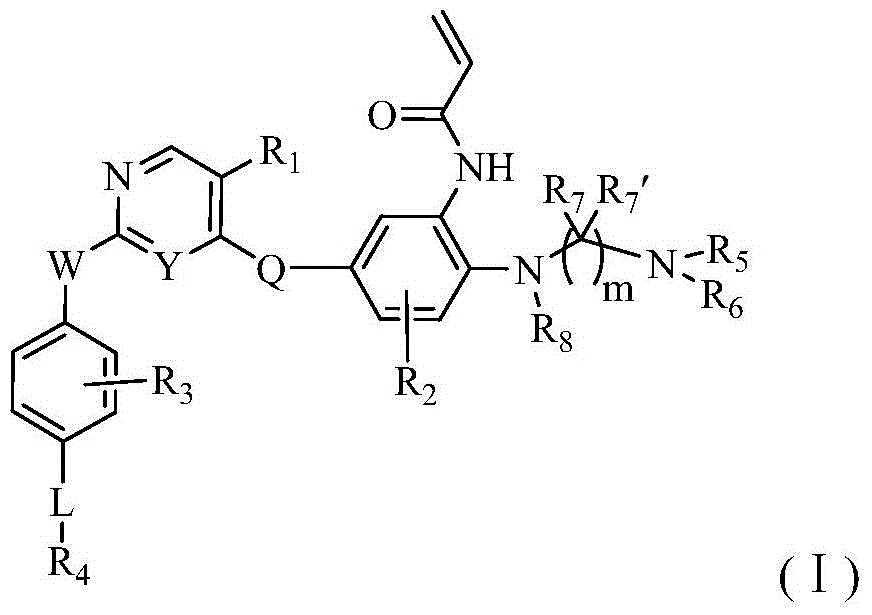
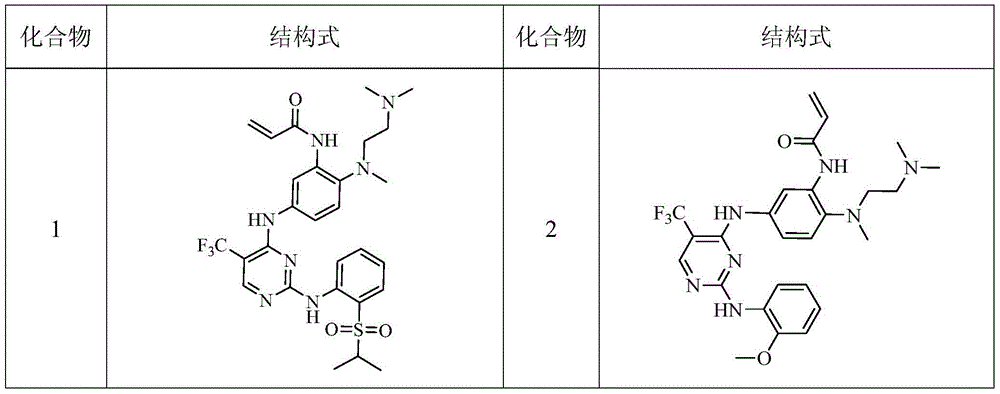
Heterocyclic derivate tyrosine kinase inhibitor
A compound and cyclic group technology, applied in the field of heterocyclic derivative tyrosine kinase inhibitors, can solve the problems of incomplete mechanism, change of ATP affinity of EGFR kinase region, loss of tumor cell killing effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0215] Preparation of Example 1 tert-butyl (4-bromo-3-nitrophenyl) carbamate
[0216]
[0217] Dissolve 3-nitro-4-bromoaniline (5.0g, 23mmol), di-tert-butyl dicarbonate (7.6g, 35mmol), triethylamine (2.32g, 23mmol) in tetrahydrofuran (50mL), heat at 60°C React for 2 hours. Spin to dry the solvent, add water (50mL), extract with ethyl acetate, separate layers, dry the organic phase, concentrate, and separate by column chromatography (petroleum ether:ethyl acetate=10:1) to obtain the product (5.8g, yield 79% ).
preparation example 2
[0218] Preparation Example 2 Preparation of tert-butyl 4-((2-(dimethylamino)ethyl)(methyl)amino)-3-nitrophenyl)carbamate
[0219]
[0220] Tert-butyl (4-bromo-3-nitrophenyl) carbamate (5.8g, 18.3mmol), N 1 ,N 1 ,N 2 - Trimethylethyl-1,2-diamine (3.73g, 36.6mmol), potassium carbonate (5.05g, 36.6mmol) were added to N,N-dimethylacetamide (75mL), 100°C oil bath 8 hours. Ethyl acetate (500 mL) was added, washed with saturated brine (500 mL), separated, the organic phase was dried, concentrated, and column chromatography (dichloromethane:methanol=10:1) gave the product (1.87 g, yield 30%) .
preparation example 3
[0221] Preparation Example 3 Preparation of tert-butyl (3-amino 4-((2-(dimethylamino) ethyl) (methyl) amino)-phenyl) carbamate
[0222]
[0223] Dissolve tert-butyl 4-((2-(dimethylamino)ethyl)(methyl)amino)-3-nitrophenyl)carbamate (1.87 g, 5.5 mmol) in methanol (25 mL) , a palladium carbon catalyst (187 mg) was added to replace the hydrogen, and the mixture was stirred at room temperature for 2 hours. Suction filtration, washing with a small amount of methanol, concentration of the organic phase, and column chromatography (dichloromethane:methanol=10:1) gave the product (1.4g, yield 82%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com