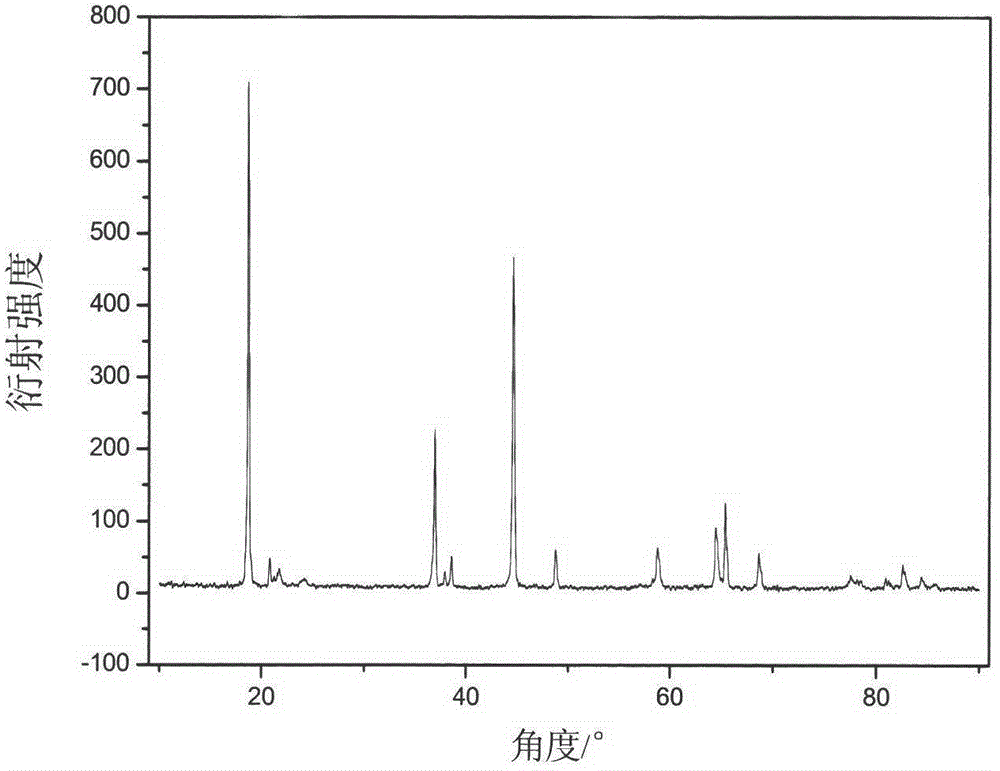
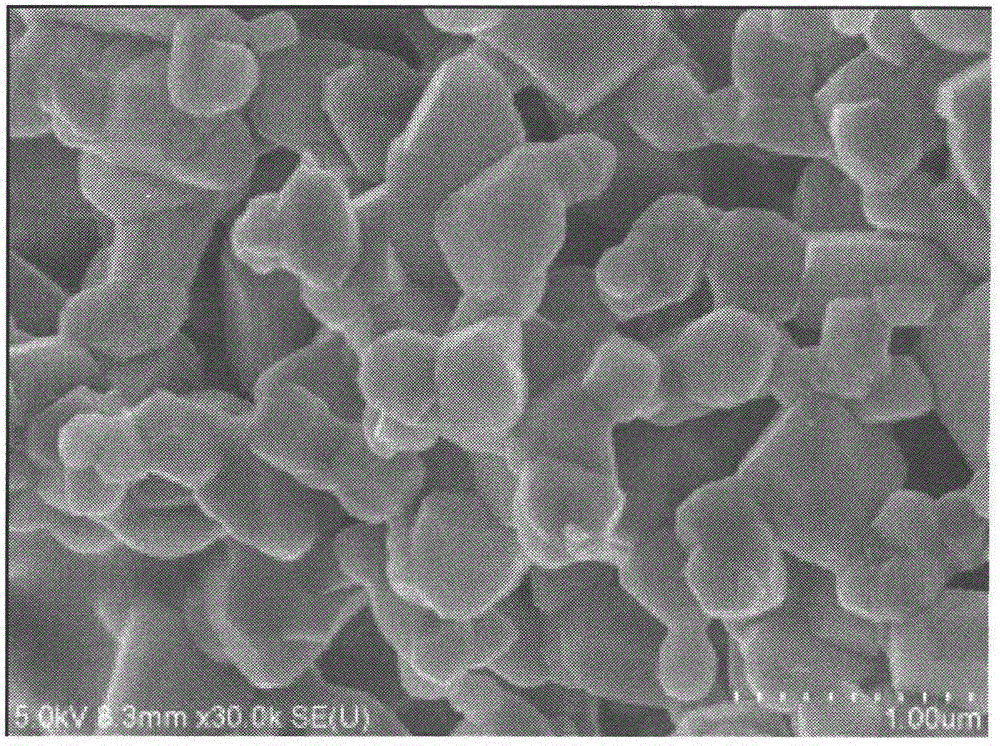
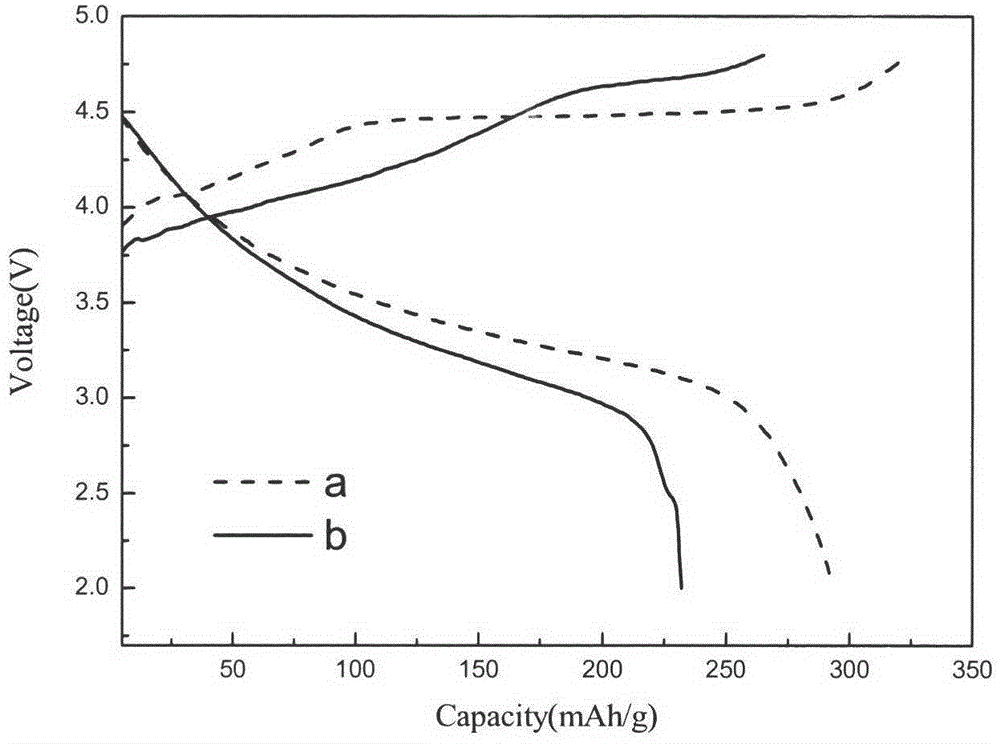
Synthesis of modified lithium-rich layered positive electrode material doped with anions of F<->, Cl<-> and Br<->
A lithium-rich cathode material and cathode material technology, applied in battery electrodes, electrical components, circuits, etc., can solve the problems of low rate performance and first Coulombic efficiency, and achieve superior electrochemical performance, increased specific surface area, and high capacity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0024] Example 1: Lithium nitrate, nickel nitrate, manganese acetate, and cobalt nitrate are weighed and dissolved in deionized water in a molar ratio of 1.26:0.15:0.55:0.1, and tartaric acid equivalent to the total molar number of metal salts (0.8mol) is added Mix the aqueous solution evenly, adjust the pH of the solution to 7 with concentrated ammonia water, and stir it in a constant temperature water bath at 70°C at a speed of 350 rpm for about 14 hours until it becomes gelatinous. The gel was baked in an oven at 90°C for 15 hours, and then placed in a muffle furnace and heated to 350°C for 8 hours for pre-sintering to obtain a precursor. After cooling and grinding, it was placed in a muffle furnace and heated to 850°C for calcination for 20 hours. , cooled and ground to obtain the final product Li[Li 0.2 Ni 0.15 mn 0.55 co 0.1 ]O 2 .
[0025] With Li[Li 0.2 Ni 0.15 mn 0.55 co 0.1 ]O 2 The positive electrode material is assembled into a half-battery, and the disch...
Embodiment example 2
[0026] Implementation case two: Lithium acetate, nickel acetate, manganese acetate, cobalt nitrate, and lithium chloride are weighed and dissolved in deionized water in a molar ratio of 1.23: 0.15: 0.55: 0.1: 0.03, and added with the total moles of metal salt ( 0.8 mol) of tartaric acid aqueous solution, mixed evenly, the pH value of the solution was adjusted to 8 with ammonia water, and stirred at a speed of 400 rpm in a constant temperature water bath at 90°C for about 12 hours until gelatinous. The gel was baked in an oven at 120°C for 12 hours, then placed in a muffle furnace and heated to 500°C for 6 hours for pre-sintering to obtain a precursor, cooled and ground, then placed in a muffle furnace and heated to 850°C for calcination for 12 hours , cooled and ground to obtain the final product Li[Li 0.2 Ni 0.15 mn 0.55 co 0.1 ]O 1.97 Cl 0.03 .
[0027] With Li[Li 0.2 Ni 0.15 mn 0.55 co 0.1 ]O 1.97 Cl 0.03 The positive electrode material is assembled into a half ...
Embodiment example 3
[0028]Implementation case three: Lithium nitrate, nickel acetate, manganese nitrate, cobalt acetate, and lithium chloride are weighed and dissolved in deionized water in a molar ratio of 1.21: 0.15: 0.55: 0.05: 0.05, and added with the total moles of metal salt ( 0.8mol) equivalent citric acid aqueous solution, mix evenly, adjust the pH value of the solution to 7 with ammonia water, stir at a speed of 500 rpm in a constant temperature water bath at 80°C for about 10 hours to gel. The gel was baked in an oven at 120°C for 12 hours, and then placed in a muffle furnace and heated to 500°C for 6 hours for pre-sintering to obtain a precursor. After cooling and grinding, it was placed in a muffle furnace and heated to 900°C for calcination for 12 hours. , cooled and ground to obtain the final product Li[Li 0.2 Ni 0.15 mn 0.55 co 0.1 ]O 1.95 Cl 0.05 .
[0029] With Li[Li 0.2 Ni 0.15 mn 0.55 co 0.1 ]O 1.95 Cl 0.05 The positive electrode material is assembled into a half ba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com