Medical adhesive made from medical polymer material and preparation method of medical adhesive
A technology of adhesives and mixtures, applied in surgical adhesives, applications, medical science and other directions, can solve the problems of unsatisfactory curing speed, unstable molded products, and human body irritation, etc., to improve product stability and curing speed. Moderate, minimal skin irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
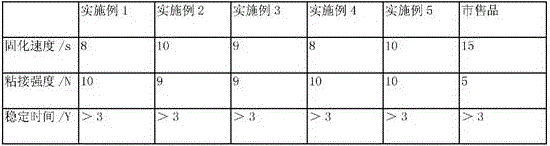
Examples
Embodiment 1
[0016] A medical adhesive, comprising in parts by weight: 3 parts of polyacrylate, 2 parts of tragacanth gum, 1 part of polymethacrylate, 2 parts of squalane, 3 parts of benzoyl peroxide, trirosin glycerol 4 parts of acid ester, 2 parts of dimethyl sebacate, 1 part of hydroquinone, 3 parts of terephthalate, 2 parts of dioctyl phthalate, 1 part of tricalcium phosphate, 3 parts of sodium alginate .
[0017] The preparation method of above-mentioned medical adhesive, comprises the following steps:
[0018] Step 1: Pour polyacrylate, tragacanth, polymethacrylate and squalane into the stirred tank, the vacuum of the stirred tank is 0.08MPa, stir evenly, heat the stirred tank to 100°C, and then add over Oxidize benzoyl and glycerol triabietate, stir evenly, and cool to room temperature to obtain mixture I;
[0019] Step 2, pour dimethyl sebacate, hydroquinone, terephthalic acid, dioctyl phthalate, tricalcium phosphate and sodium alginate into the stirring tank, the vacuum degree o...
Embodiment 2
[0022] A medical adhesive, comprising in parts by weight: 4 parts of polyacrylate, 3 parts of tragacanth gum, 2 parts of polymethacrylate, 3 parts of squalane, 5 parts of benzoyl peroxide, trirosin glycerol 6 parts of acid ester, 3 parts of dimethyl sebacate, 2 parts of hydroquinone, 5 parts of terephthalate, 4 parts of dioctyl phthalate, 2 parts of tricalcium phosphate, 4 parts of sodium alginate .
[0023] The preparation method of above-mentioned medical adhesive, comprises the following steps:
[0024] Step 1: Pour polyacrylate, tragacanth, polymethacrylate and squalane into the stirring tank, the vacuum degree of the stirring tank is 0.09MPa, stir evenly, heat the stirring tank to 110°C, and then add over Oxidize benzoyl and glycerol triabietate, stir evenly, and cool to room temperature to obtain mixture I;
[0025] Step 2, pour dimethyl sebacate, hydroquinone, terephthalic acid, dioctyl phthalate, tricalcium phosphate and sodium alginate into the stirring tank, and th...
Embodiment 3
[0028] A medical adhesive, comprising in parts by weight: 7 parts of polyacrylate, 5 parts of tragacanth gum, 4 parts of polymethacrylate, 5 parts of squalane, 9 parts of benzoyl peroxide, trirosin glycerol 7 parts of acid ester, 6 parts of dimethyl sebacate, 4 parts of hydroquinone, 7 parts of terephthalate, 6 parts of dioctyl phthalate, 3 parts of tricalcium phosphate, 7 parts of sodium alginate .
[0029] The preparation method of above-mentioned medical adhesive, comprises the following steps:
[0030] Step 1: Pour polyacrylate, tragacanth, polymethacrylate and squalane into the stirring tank, the vacuum degree of the stirring tank is 0.1MPa, stir evenly, heat the stirring tank to 100°C, and then add over Oxidize benzoyl and glycerol triabietate, stir evenly, and cool to room temperature to obtain mixture I;
[0031] Step 2, pour dimethyl sebacate, hydroquinone, terephthalic acid, dioctyl phthalate, tricalcium phosphate and sodium alginate into the stirring tank, and the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| curing time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com