Method for preparing water-soluble glowing metal clusters of platinum, gold, silver and copper and application
A metal platinum, water-soluble technology, applied in the field of preparation of luminescent metal clusters, can solve the problems of limited widespread adoption, complex preparation process, lack of versatility, etc., and achieve the effects of fast conditions, uniform size distribution, and good luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Preparation of histidine-stabilized platinum clusters:
[0018] a. Add 1 mL of 0.01 mol / L platinum chloride aqueous solution to a mixture of 1 mL of 0.1 mol / L histidine aqueous solution and 8 mL of deionized water and stir for 30 minutes at a stirring speed of 300 rpm to obtain a mixed solution;
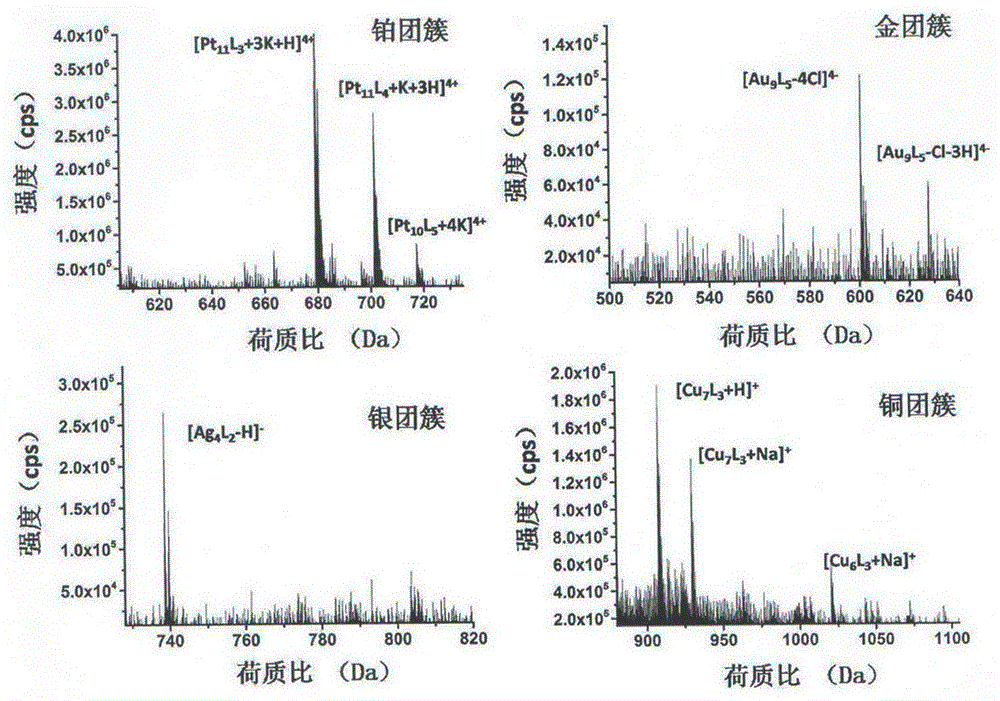
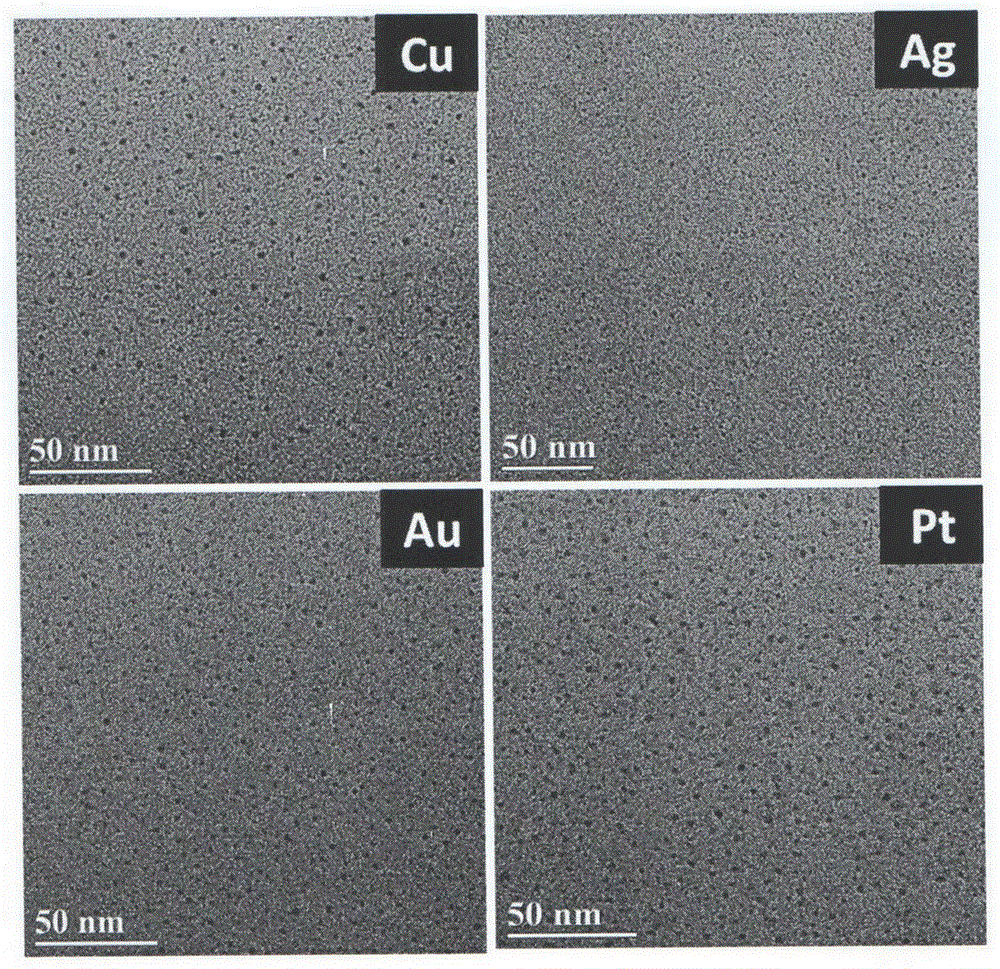
[0019] b. Add 0.1 mol / L ascorbic acid to the mixed solution in step a, continue to stir and react at a temperature of 40° C. for 10 hours, and the stirring speed is 300 rpm to obtain platinum clusters with an average particle size of 1.9 nm;
[0020] Detection of iron ions:
[0021] Prepare a group of Fe with different concentrations 2 (SO 4 ) 3 Aqueous solution, pH=4, the concentration of the prepared solution is from 0.1mmol·L -1 to 100mmol·L -1 ranging;
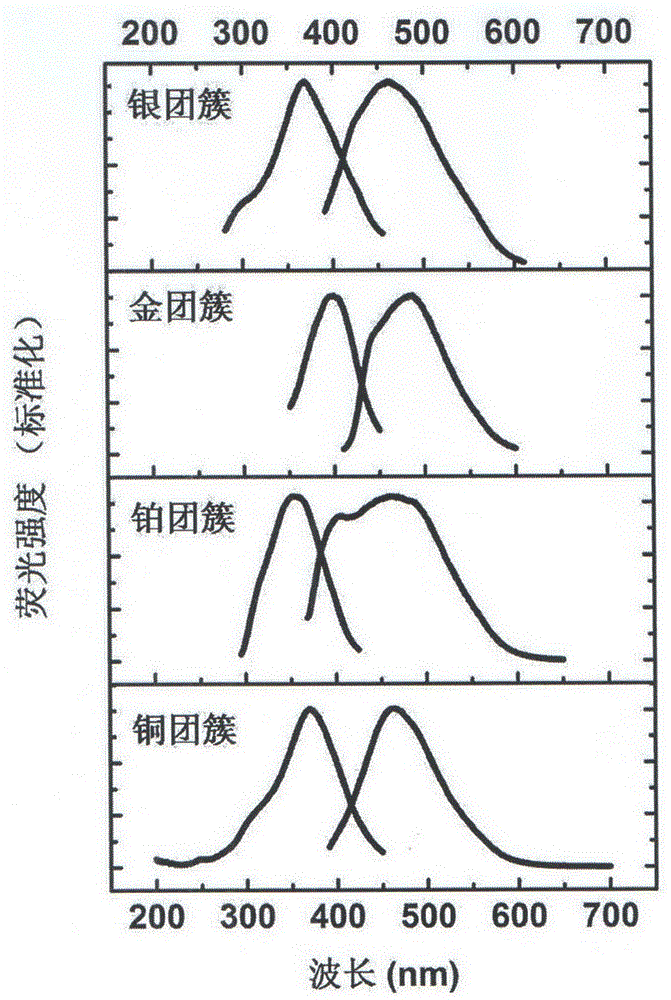
[0022] Take a group of 2mL prepared platinum cluster solutions, add different concentrations of Fe 2 (SO4) 3 1 μL of aqueous solution, and record the fluorescence emission spectrum of platinum clusters after each a...
Embodiment 2
[0024] Preparation of histidine-stabilized gold clusters:
[0025] a. Add 1 mL of 0.01 mol / L chloroauric acid aqueous solution to a mixture of 1 mL of 0.1 mol / L histidine aqueous solution and 8 mL of deionized water and stir for 1 minute at a stirring speed of 300 rpm to obtain a mixed solution;
[0026] b. Add 0.1 mol / L ascorbic acid to the mixed solution in step a, continue stirring and reacting at a temperature of 25° C. for 15 minutes at a stirring speed of 300 rpm to obtain gold clusters with an average particle size of 1.8 nm;
[0027] Detection of iron ions:
[0028] Prepare a group of Fe with different concentrations 2 (SO 4 ) 3 Aqueous solution, pH=4, the concentration of the prepared solution is from 0.1mmol·L -1 to 100mmol·L -1 ranging;
[0029] Take a group of 2mL prepared gold cluster solutions, add different concentrations of Fe 2 (SO 4 ) 3 1 μL of aqueous solution, and record the fluorescence emission spectrum of gold clusters after each addition of fer...
Embodiment 3
[0031] Preparation of histidine-stabilized silver clusters:
[0032] a. Add 1 mL of 0.01 mol / L silver nitrate aqueous solution to a mixture of 1 mL of 0.1 mol / L histidine aqueous solution and 8 mL of deionized water and stir for 60 minutes at a stirring speed of 300 rpm to obtain a mixed solution;
[0033] b. Add 0.1mol / L ascorbic acid to the mixed solution in step a, continue to stir and react at a temperature of 35°C for 4 hours, and the stirring speed is 300rpm, to obtain silver clusters with an average particle size of 1.3nm;
[0034] Detection of iron ions:
[0035] Prepare a group of Fe with different concentrations 2 (SO 4 ) 3 Aqueous solution, pH=4, the concentration of the prepared solution is from 0.1mmol·L -1 to 100mmol·L -1 ranging;
[0036] Take a group of 2mL prepared silver cluster solutions, add different concentrations of Fe 2 (SO4) 31 μL of aqueous solution, and record the fluorescence emission spectrum of silver clusters after each addition of ferric...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com