Method for preparing bupropion hydrochloride
A technology of bupropion hydrochloride and acetone, which is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of unfavorable commercial production, increase of production cost, pollution of the environment, etc., and achieve low cost and lighten Damage, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
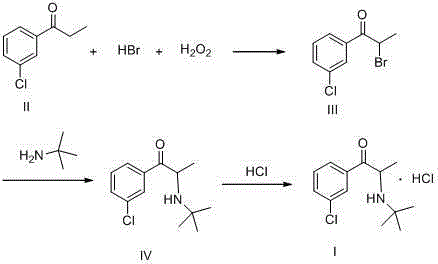
[0029] Take 33.7g of m-chloropropiophenone and 100g of dichloromethane in a 500mL three-necked flask, stir to dissolve, and add 22mL of 30% hydrogen peroxide in a water bath with temperature controlled at 20°C. After the addition is complete, control the reaction temperature at 20-25°C and add 0.5mL of 48% hydrobromic acid solution dropwise. After the reaction is initiated, keep the reaction temperature at 20-25°C and add the remaining hydrobromic acid dropwise, add a total of 45ml, and stir for 1 hour. Add 120g of water to wash, and stir for 20min, let it stand for 30min, separate the organic layer, transfer it to a three-necked flask again, and distill under reduced pressure in a water bath at 50°C until no fraction flows out to obtain 49.1g of a light yellow liquid with an HPLC purity of 98.5% .
[0030] Put 49.1g of the above bromide (light yellow liquid), 90mL of dichloromethane into a 500mL three-neck flask, stir to dissolve, add 49mL of tert-butylamine at a temperature ...
Embodiment 2
[0033] Take 33.7g of m-chloropropiophenone and 100g of acetonitrile in a 500mL three-necked flask, stir to dissolve, add 22mL of 30% hydrogen peroxide in a water bath with temperature controlled at 20°C. After the addition is complete, control the reaction temperature at 20-25°C and add 0.5mL of 48% hydrobromic acid solution dropwise. After the reaction is initiated, keep the reaction temperature at 20-25°C and add the remaining hydrobromic acid dropwise, add a total of 45ml, and stir for 1 hour. Add 120g of water to wash, and stir for 20min, let it stand for 30min, separate the organic layer, transfer it to a three-necked flask again, distill under reduced pressure in a water bath at 50°C until no fraction flows out, and obtain 48.5g of a light yellow liquid with a HPLC purity of 98.2% .
[0034] Put 48.5g of the above bromide (light yellow liquid), 90mL of dichloromethane into a 500mL three-neck flask, stir to dissolve, add 48.5mL of tert-butylamine at a temperature of 20-25...
Embodiment 3
[0037] Take 33.7g of m-chloropropiophenone and 100g of dichloromethane in a 500mL three-neck flask, stir to dissolve, and add 22mL of 30% hydrogen peroxide in a water bath with temperature controlled at 20°C. After the addition, control the reaction temperature at 20-25°C and add 0.5mL 48% hydrobromic acid solution dropwise. After the reaction is initiated, keep the reaction temperature at 20-25°C and add the remaining hydrobromic acid dropwise, add a total of 35ml, and stir for 1 hour . Add 120 g of water to wash, stir for 20 min, let stand for 30 min, separate the organic layer, transfer it to a three-necked flask, and distill under reduced pressure in a 50°C water bath until no fraction flows out. 43.7 g of a light yellow liquid was obtained with an HPLC purity of 98.4%.
[0038]Put 43.7g of the above bromide (light yellow liquid), 90mL of dichloromethane into a 500mL three-neck flask, stir to dissolve, add 44mL of tert-butylamine at a temperature of 20-25°C, after the add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com