A kind of recombinant antiviral protein and its preparation method and application
An antiviral and protein technology, applied in antiviral agents, peptide/protein components, chemical instruments and methods, etc., can solve the problems of low specificity, high toxicity and unstable preventive effect of chemical antiviral drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1 Construction of recombinant antiviral protein P9-ISG20 expression vector
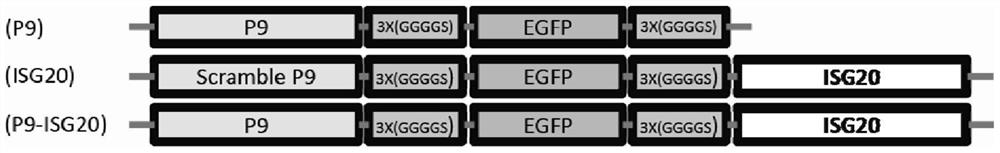
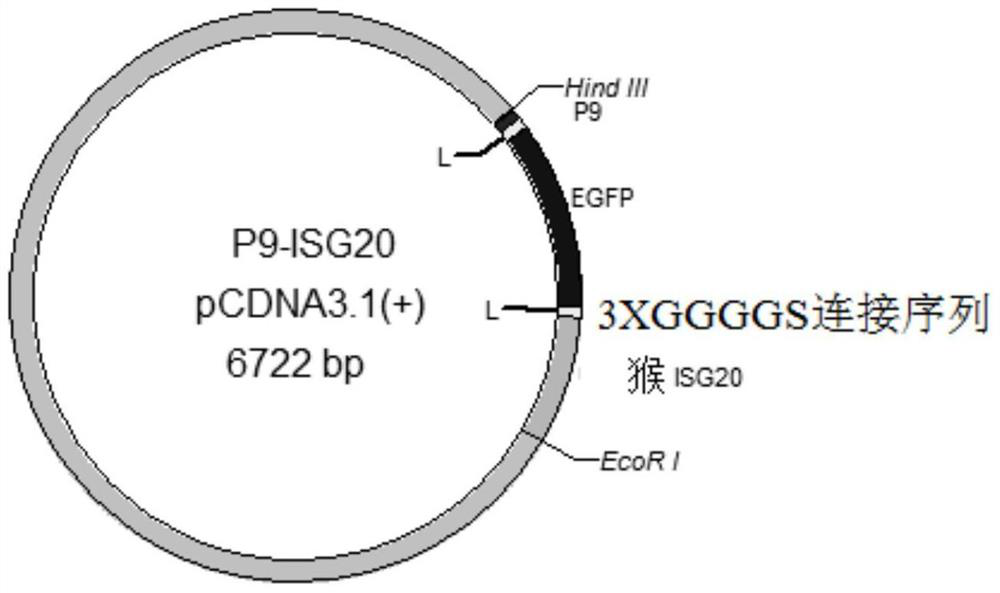
[0053] In this example, a new clone P9-Linker-EGFP-Linker-ISG20 (P9-ISG20) was constructed, which fused P9 and ISG20 and was constructed on the pCDNA3.1(+) eukaryotic expression vector. For fluorescence detection experiments, the EGFP gene is connected to the N-terminal end of ISG20 through two linking sequences 3╳(GGGGS). The specific cloning design is as follows figure 1 shown. The build method is as follows:
[0054] 1) Construction of pcDNA-P9 plasmid vector
[0055] The P9 forward and reverse primers were annealed to construct the P9 gene, and the P9 forward primer and reverse primer were as follows (the nucleotide sequence of which is shown in SEQ ID No. 7-8 of the sequence table):
[0056] P9 forward primer:
[0057] 5'-AGCTTCACATCAGGCTGACATTGAGCAGAAATAAGAACACCG-3'
[0058] P9 reverse primer:
[0059] 5'-AATTCGGTGTTCTTATTTCTGCTCAATGTCAGCCTGATGTGA-3'
[0060] Use the ann...
Embodiment 2
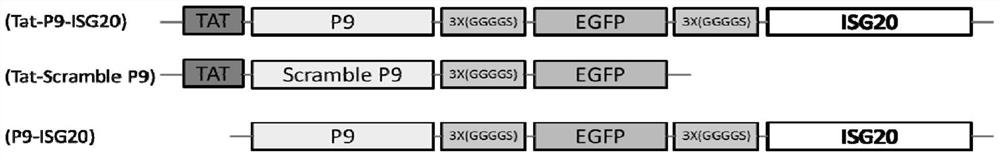
[0078] Embodiment 2 Construction of recombinant antiviral protein TAT-P9-ISG20 expression vector
[0079] Using the pcDNA-P9-EGFP-ISG20 plasmid constructed in Example 1 as a template, design primers containing the TAT gene sequence, use a homologous recombination kit to connect TAT into the N-terminal of the P9-EGFP-ISG20 gene by homologous recombination, and amplify The amplification primers are as follows (the nucleotide sequence is shown in SEQ ID No.20-21 in the sequence table):
[0080] Upstream primers:
[0081] 5'-AAGCTTTATGGCAGGAAGAAGCGGAGACAGCGACGAAGACACATCA-3'
[0082] Downstream primers:
[0083]5'-GAATTCTCAGTCTGACACAGCCAGGCGGGGCAGCCCTCGGCGG-3'
[0084] The amplified TAT-P9-EGFP-ISG20 gene was purified and recovered, and the target gene TAT-P9-EGFP-ISG20 gene was cloned into the cell-free expression vector pIVEX-HisTag by the homologous recombination method in Example 1 to obtain the expression vector pIVEX TAT- P9-EGFP-ISG20. pIVEX-His Tag is an expression pla...
Embodiment 3
[0086] Example 3 Inhibition of recombinant antiviral protein P9-ISG20 on PRRSV virus infection
[0087] (1) Cultivate MARC-145 cells, spread the cells to 24-well cell culture plates, and wait until the cells grow to 70%;
[0088] (2) Transfect the recombinant antiviral protein P9-ISG20 expression vector constructed in Example 1, the P9 and ISG20 expression vectors and empty load as a control; the specific steps are as follows: 50 microliters of 1640 RIPM serum-free medium and 2 microliters of transfection Dyeing reagent (Liposome 2000) was mixed evenly. Mix 50 microliters of 1640 RIPM serum-free medium with 0.5 micrograms of expression plasmid vectors evenly. Mix the transfection reagent and the expression plasmid vector evenly, let stand at room temperature for 5 minutes, then directly add to the cell well, and continue to incubate in a 37°C incubator for 24 hours;
[0089] (3) 24 hours after transfection, the liquid in the cell wells was discarded, and the cells were washe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com