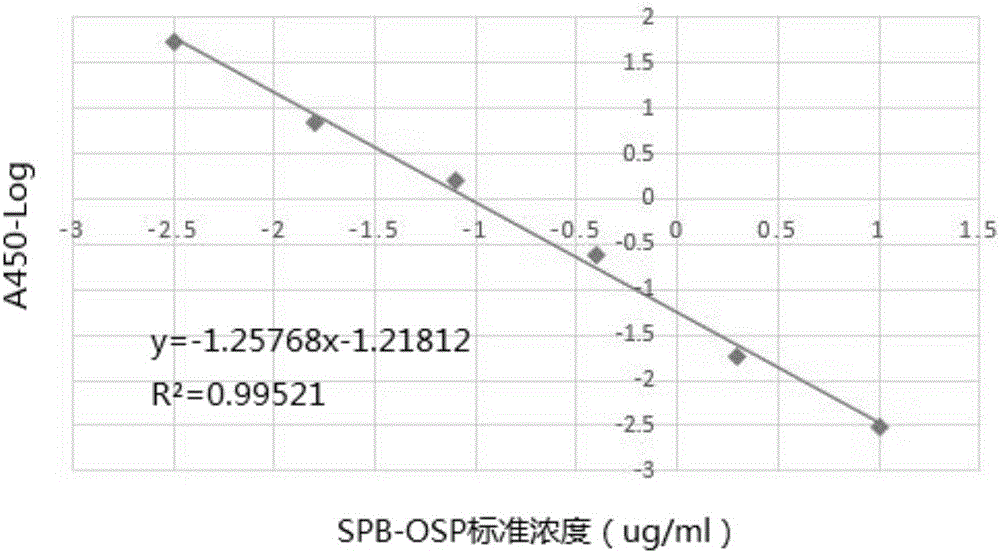
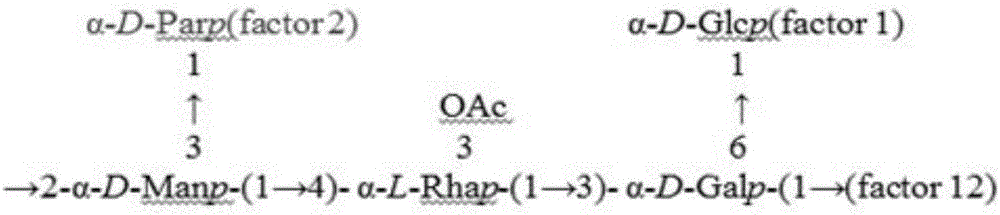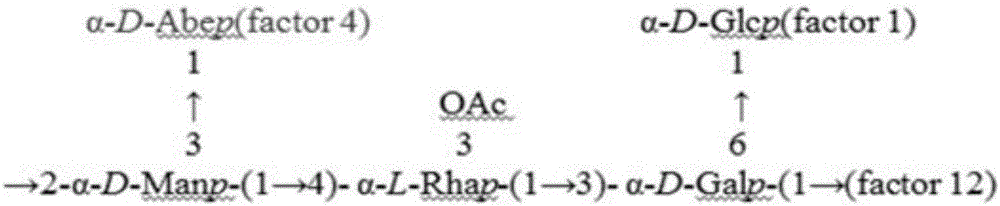Hepatitis-B-paratyphoid-fever-resistant O-SP monoclonal antibody, hybridoma cell strain and application
A hybridoma cell line, paratyphoid B technology, applied in the direction of anti-bacterial immunoglobulin, chemical instruments and methods, biochemical equipment and methods, etc., can solve the problem of inability to effectively distinguish between paratyphoid A and paratyphoid B , Monoclonal antibody screening is difficult, specific sites are not obvious, etc., to achieve the effect of high sensitivity, simple operation, and avoid complicated operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Establishment of anti-paratyphoid B O-SP monoclonal antibody hybridoma cell line WV-SPB-11
[0035] Unless otherwise specified, the vaccines and polysaccharides involved in this example can be prepared according to Part Three of the Pharmacopoeia of the People's Republic of China 2015 Edition or obtained from the market.
[0036] 1.1 Source of antigen
[0037] Paratyphoid B O-SP conjugate vaccine (lyophilized) (batch number 20131015) was provided by Yuxi Watson Biotechnology Co., Ltd.
[0038] 1.2 Source of mice
[0039] Female, 6-8-week-old BALB / c mice, SPF grade (Specific pathogen Free, SPF), 15-20 g, were purchased from Guangdong Medical Experimental Animal Center (permit number SCXK (Guangdong) 20130002).
[0040] 1.3 Source and maintenance of myeloma cells
[0041] Myeloma cells SP2 / 0 were purchased from the Kunming Cell Bank of the Type Culture Collection Center of the Chinese Academy of Sciences (KCB92021YJ)
[0042] 1.4 Preparation of hybridoma cell...
Embodiment 2
[0072] Embodiment 2: Preparation of monoclonal antibody of type B paratyphoid hybridoma cell line
[0073] 2.1 Mice were inoculated with hybridoma cells
[0074] Normal 2-month-old BALB / c mice were selected and injected with liquid paraffin 0.5ml / mouse 1 day in advance. The cultured paratyphoid hybridoma cells were centrifuged to discard the supernatant, and the serum-free medium was added and injected into the peritoneal cavity of mice. Hybridoma cells proliferate in the peritoneal cavity of mice and produce and secrete monoclonal antibodies. About 1-2 weeks, the mouse abdomen can be seen to expand. A large amount of monoclonal antibodies can be obtained by extracting ascitic fluid with a syringe.
[0075] 2.2 Monoclonal antibody titer test Ascites titer test, double dilution of collected ascites: 1:10 3 , 1: 2×10 3 , 1:4×10 3 , 1:8×10 3 、1:1.6×10 4 、1:3.2×10 4 、1:6.4×10 4 、1:1.28×10 5 、1:2.56×10 5 , Each concentration was repeated twice, and the titer of the mono...
Embodiment 3
[0078] Example 3: Establishment of ELISA detection method for monoclonal antibody to paratyphoid B O-SP
[0079] 3.1 Configuration of the main solution
[0080] Coating buffer (0.1mol / L, hydrochloride buffer at pH 9.6): Na2CO3·10H2O0.86g; NaHCO30.586g; add sterilized water to 200ml.
[0081] PBS diluent (0.01mol / L, PBS buffer solution with pH7.4): 8g NaCl; 0.2g KCl; 2.9g Na2HPO4·12H2O; 0.2g KH2PO4; add sterilized water to 100ml.
[0082] Washing solution (0.01mol / L, PBST at pH7.4): 1000ml of 1PBS buffer; 0.5ml of Tween-20.
[0083] Blocking solution (5% skimmed milk powder PBS diluent): 1 g skimmed milk powder; 20 ml PBS diluent.
[0084] Chromogenic solution: substrate solution 10ml; TMB 0.5ml; H 2 o 2 32 μl.
[0085] Stop solution (2mol / L H2SO4): concentrated sulfuric acid (18mol / L 98%) 22.2ml; sterilized water 177.8ml.
[0086] 3.2 Elisa indirect method to detect monoclonal antibody titer
[0087] 3.2.1 Coating: SPB-O-SP-20130511 (1 mg / ml) was diluted to 500 ng / ml wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com