Carbon-based nanocomposite material for electrode
A carbon-based nano-composite material technology, applied in the fields of nanotechnology, battery electrodes, and nanotechnology for materials and surface science, can solve the problems of rapid disintegration of electrode materials, reduction of material cycle capacity, and large volume changes. Achieve the effect of being beneficial to large-scale synthesis, easy to collect, and less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
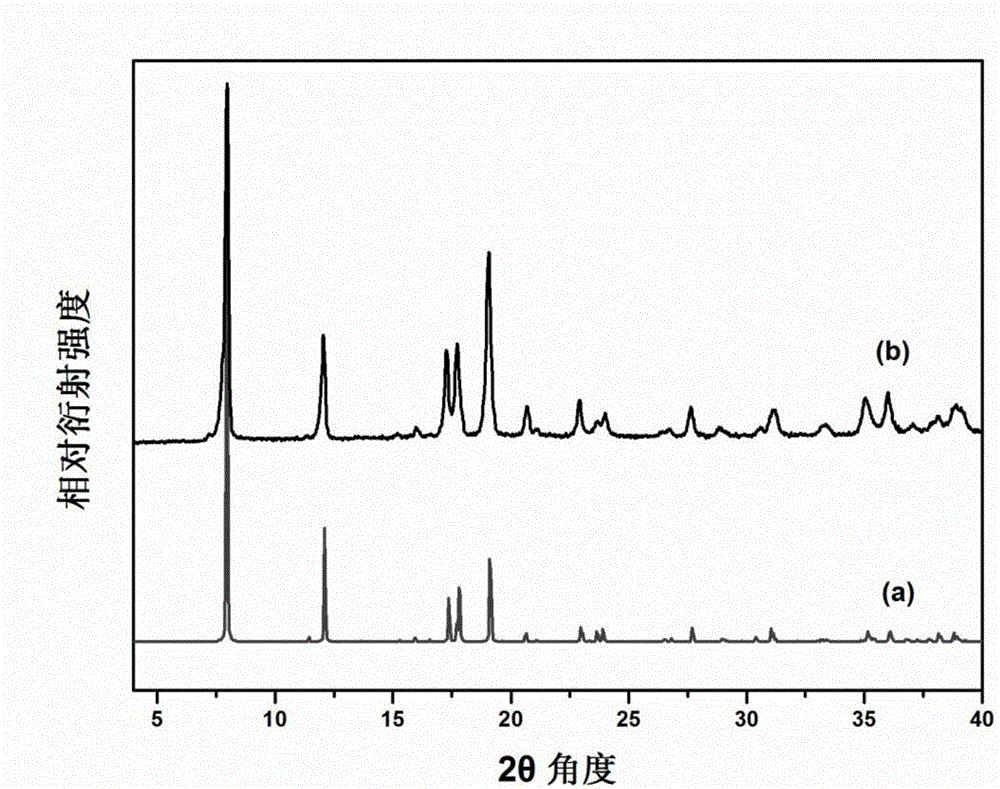
[0048] (1)Ni(L-asp)(H 2 O) 2 Preparation: NiCO 3 2Ni(OH) 2 ·xH 2 O, L-asp and H 2 O was mixed in a ratio of 1:1:200, and kept under heating and stirring at 95°C for 2.5 hours, until most of the powder was dissolved in water, then the heating and stirring were stopped. The insoluble matter in the solution was removed by filtration to obtain a clear solution, which was placed in an oven at 100°C for 1 day, and the green Ni(L-asp)(H 2 O) 2 Crystals, collected for later use.
[0049] (2)[Ni 2 (L-asp) 2 (bpy)] preparation of crystal material: Ni(L-asp)(H 2 O) 2 Add it into the mixed solution of methanol and water, and continue to add a certain amount of 4,4'-pyridine under stirring conditions after partially dissolving, so that the mass ratio of each substance in the final solution is Ni(L-asp)(H 2 O) 2 :4,4'-pyridine:methanol:water=1:4:21.7:27.4. The solution was transferred to an autoclave, sealed, and crystallized in an oven at 150° C. for 2 days. After the reactor...
Embodiment 2
[0055] Prepare [Ni by step (1) and (2) in embodiment 1 2 (L-asp) 2 (bpy)] crystalline powder material.
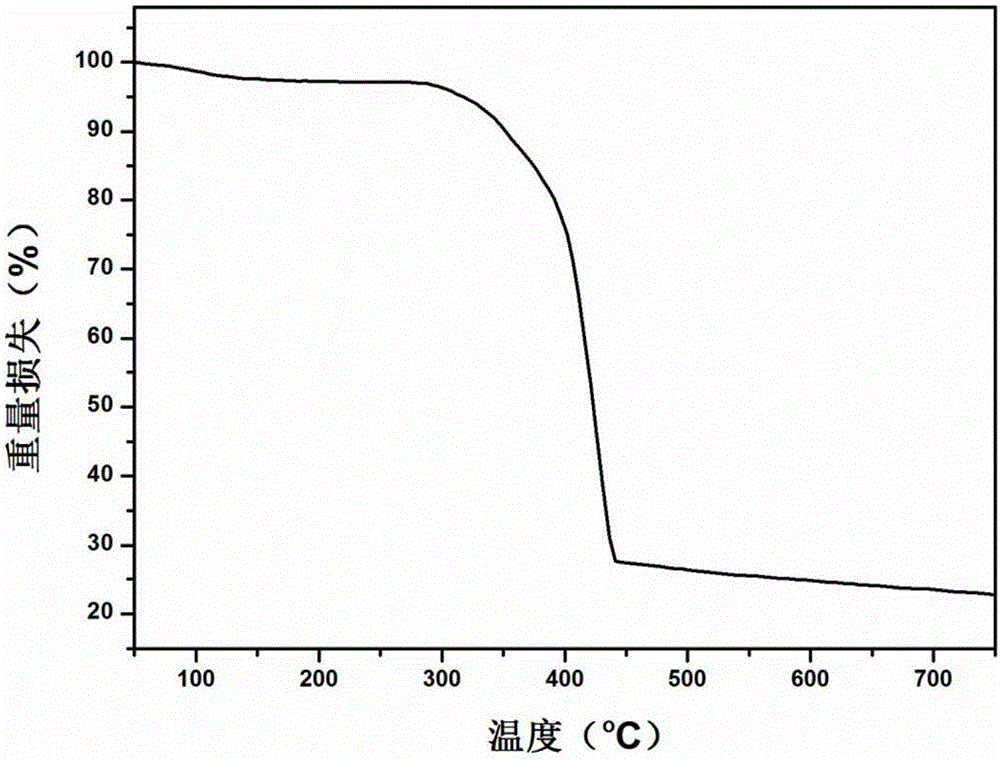
[0056] The synthesized [Ni 2 (L-asp) 2 (bpy)] The crystal powder is lightly ground until there are no larger particles and spread on the bottom of the ceramic crucible. The porcelain crucible is placed in the middle of the tube furnace, and then the internal air of the tube furnace is removed by vacuuming. Perform replacement and repeat 3 times to ensure a sufficient anaerobic environment. The flow rate of nitrogen is controlled at 80ml per minute, and the heating rate is 5°C per second. First, stay at 150°C for 2 hours, and then pyrolyze at 900°C for 5 hours. Get Metal@Carbon Composite.
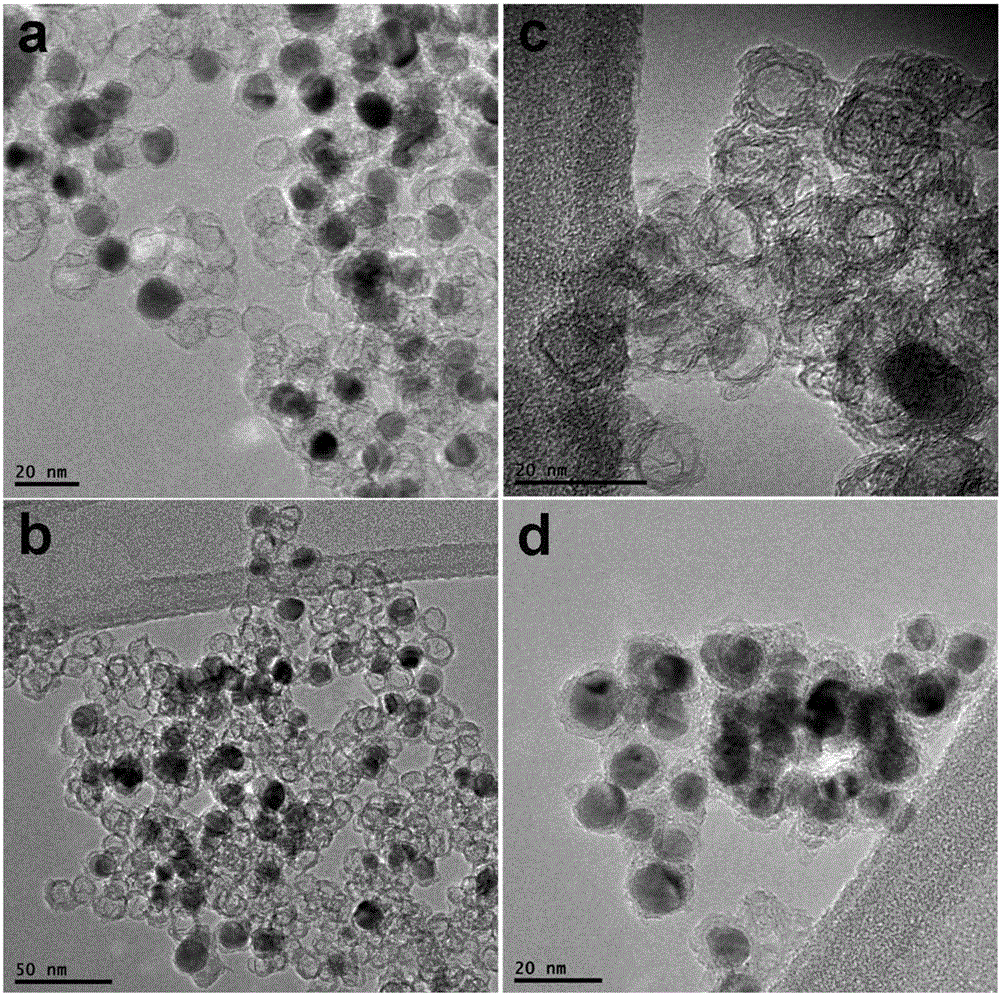
[0057] Step (4) and step (5) in Example 1 were repeated, and the carbon-based nanocomposite material used for the electrode was characterized by transmission electron microscopy and lithium ion battery performance.
Embodiment 3
[0059] Prepare [Ni by step (1) and (2) in embodiment 1 2 (L-asp) 2 (bpy)] crystalline powder material.
[0060] The synthesized [Ni 2 (L-asp) 2 (bpy)] The crystal powder is lightly ground until there are no larger particles and spread on the bottom of the ceramic crucible. The porcelain crucible is placed in the middle of the tube furnace, and then the internal air of the tube furnace is removed by vacuuming. Perform replacement and repeat 3 times to ensure a sufficient anaerobic environment. The flow rate of nitrogen is controlled at 80ml per minute, the heating rate is 5°C per second, first stay at 150°C for 2 hours, and then pyrolyze at 1100°C for 5 hours. Get Metal@Carbon Composite.
[0061] Step (4) and step (5) in Example 1 were repeated, and the carbon-based nanocomposite material used for the electrode was characterized by transmission electron microscopy and lithium ion battery performance.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com