Production method of trifluoromethoxybenzene
A technology of trifluoromethoxybenzene and production method, applied in the production of trifluoromethoxybenzene and the production field of aromatic compounds, can solve the problems of reduced yield, high cost and high consumption, achieve high recovery rate, The effect of smooth response and reduced usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0032] The present invention will be further described below through examples, but the protection scope of the present invention is not limited thereto.
[0033] The raw materials used in this example are all commercially available products. The analysis of the reaction process adopts gas chromatography analysis method.
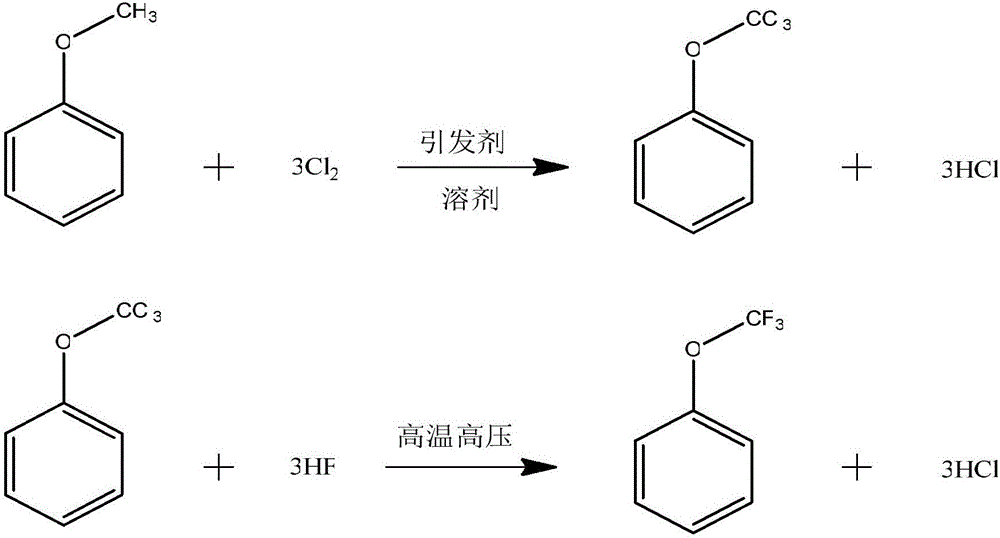
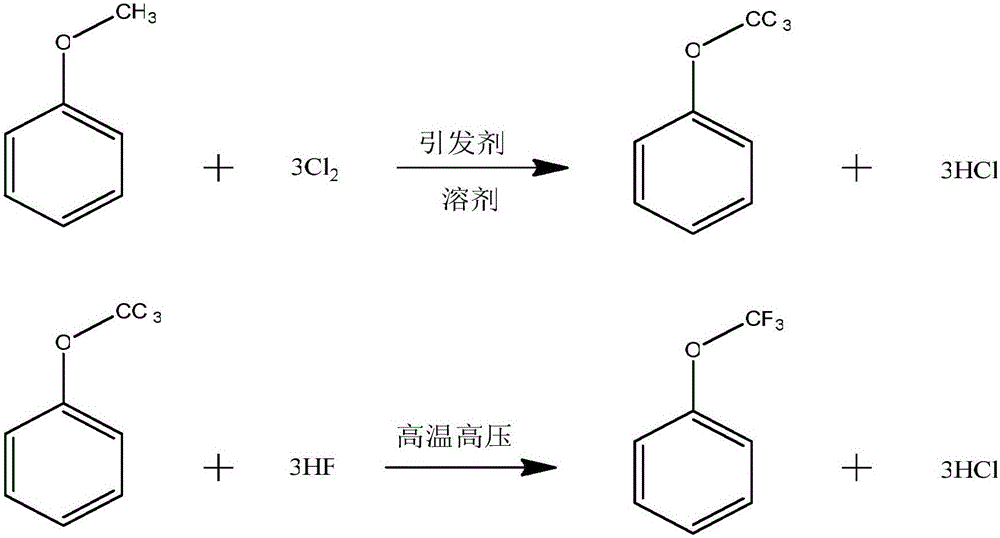
[0034] The chemical reaction formula of the present embodiment is as follows:
[0035]
Embodiment 1
[0037] 1. Chlorination reaction
[0038]Add 150g of p-chlorobenzotrifluoride solvent into a 1000ml reactor equipped with mechanical stirring, condenser, thermometer, bottom vent pipe, and dropping pipe, start stirring, heat up to 139-140°C, until the solvent refluxes, pass Chlorine gas, start dropwise adding a mixed solution of 200g of anisole, 600g of p-chlorobenzotrifluoride solvent and 10g of azobisisobutyronitrile initiator, and add dropwise at 139-140°C for about 12 hours. After the dropwise addition is completed, take a sample for analysis. When dichloromethoxybenzene is lower than 0.3%, stop the reaction and lower the temperature.
[0039] 2. Fluorination reaction
[0040] Add 1134.5g of the mixed solution obtained from the chlorination reaction into a 1L autoclave, add 133.3g of hydrogen fluoride, start stirring, raise the temperature to 95°C, and keep the reaction under a pressure of 2.8Mpa for 3-4h. Stop the reaction and take a sample for analysis.
[0041] 3. Pro...
Embodiment 2
[0044] 1. Chlorination reaction
[0045] Add 450g of p-chlorobenzotrifluoride solvent into a 3000ml reactor equipped with mechanical stirring, condenser, thermometer, bottom vent tube, and dropping tube, start stirring, and raise the temperature to 139-140 degrees Celsius to reach 450 g of p-chlorobenzotrifluoride solvent. When reflux occurs, pass chlorine gas, and start to drop a mixed solution of 600g of anisole, 1200g of p-chlorobenzotrifluoride solvent and 30g of azobisisobutyronitrile initiator, dropwise at 139-140°C for about 12 hours, dropwise Sampling and analysis after completion, when dichloromethoxybenzene is less than 0.3%, stop the reaction and cool down.
[0046] 2. Fluorination reaction
[0047] Add 2811.91g of the mixed solution obtained from the chlorination reaction into the autoclave, add 400.0g of hydrogen fluoride, start stirring, raise the temperature to 90°C, and keep the reaction under 2.5Mpa pressure for 3-4h. Stop the reaction and take a sample for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com