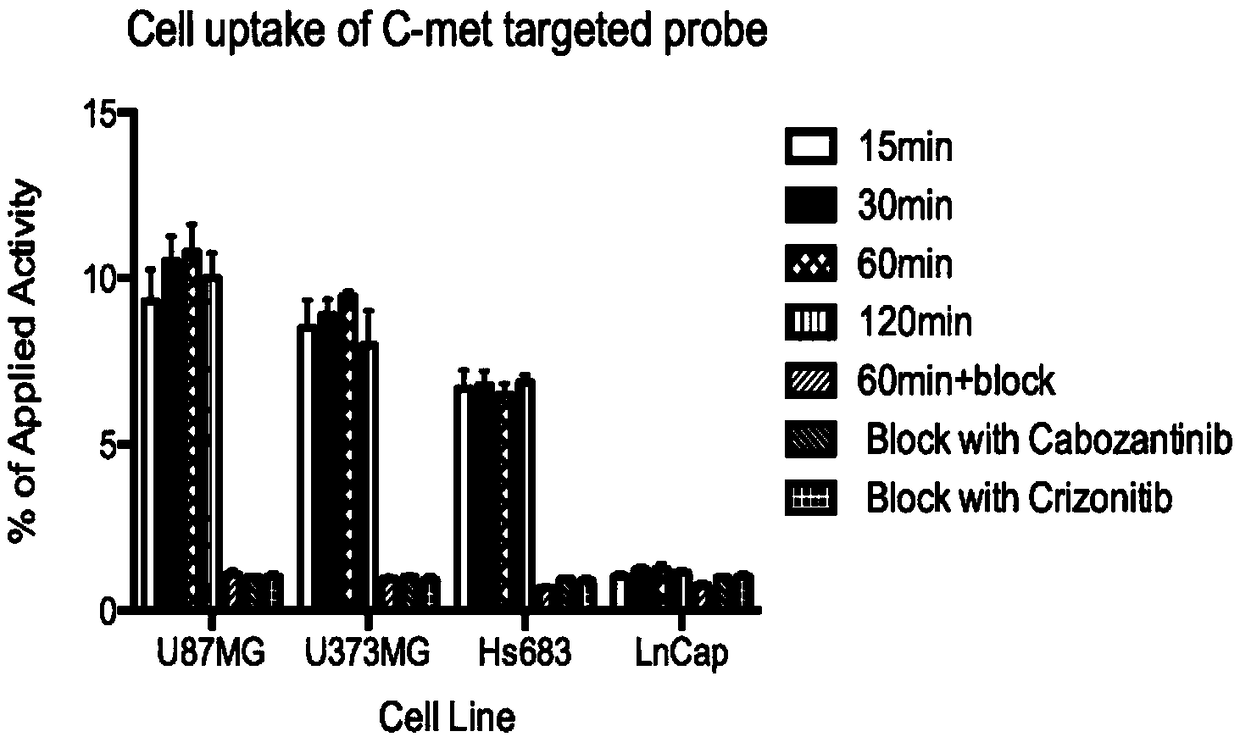
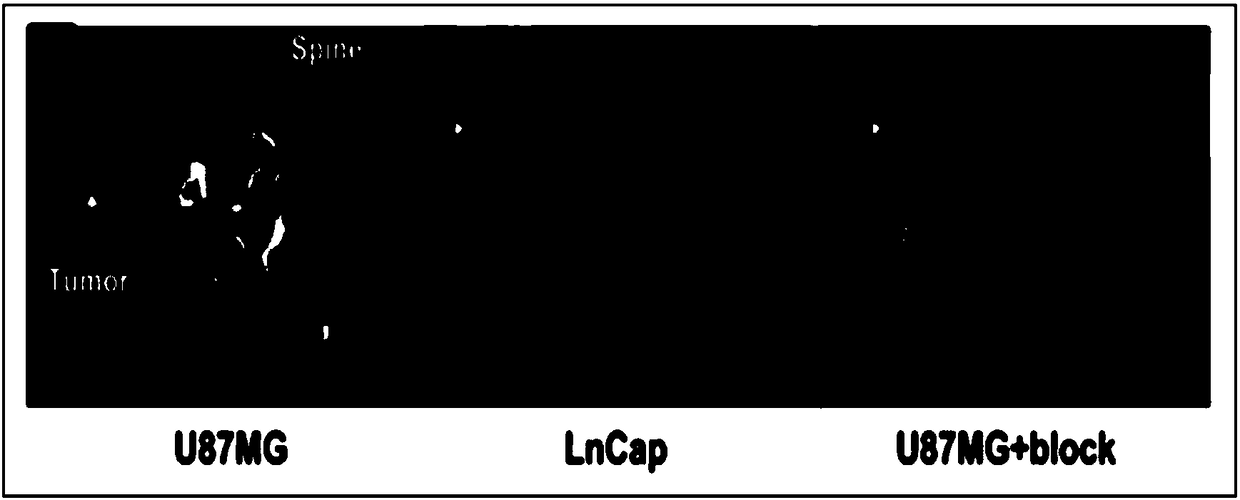
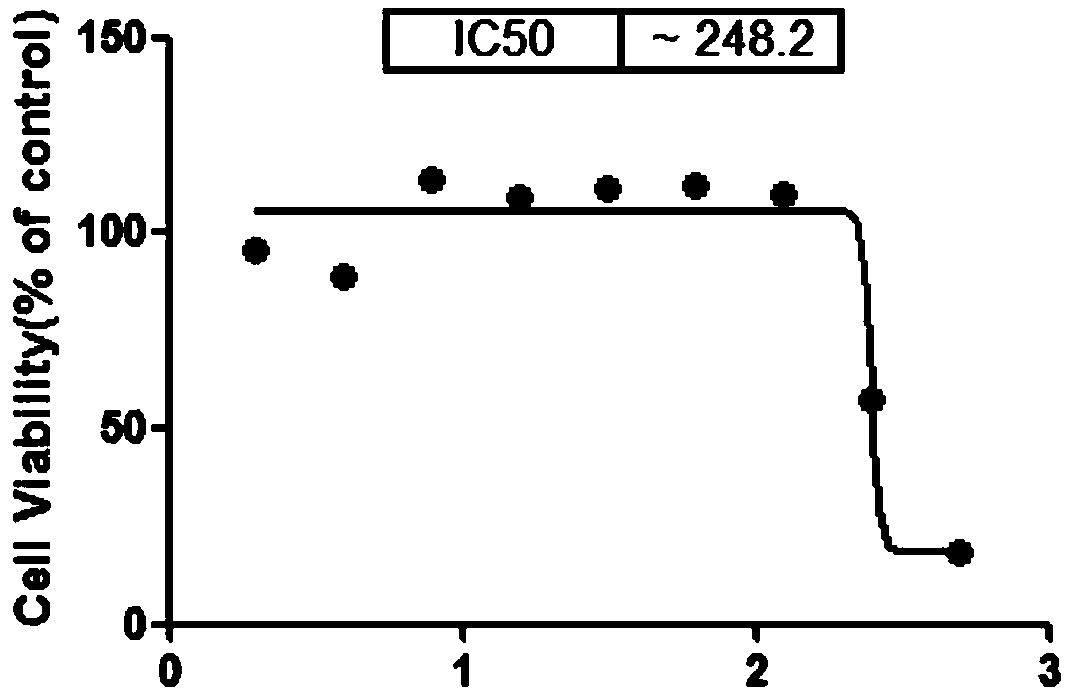
A radioactive c-met targeting affinity small molecule compound and its application
A technology of small molecule compounds and radioactivity, which is applied in the direction of radioactive carriers, radioactive preparations in vivo, heterocyclic compound isotope introduction, etc., can solve the problems of inability to realize dynamic research, unfavorable dynamic research, complicated operation, etc., and achieve good biological distribution characteristics, Good pharmacokinetic characteristics and accurate test results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] 1) 18 Synthesis and characterization of F-cMET-1 probe;
[0051]
[0052] Reagent: 4 mg cMET-1, K 18 F, K 222 / K 2 CO 3 solution ( 18 For F labeling, containing 15mgK 222 and 3.5mgK 2 CO 3 per mL), dry DMSO.
[0053] Operation process:
[0054] use standard 18 F radioactive labeling reaction apparatus for labeling. Concretely, the K 18 F(K 2 CO 3 Neutralize H 18 F produced), add 1mLK 222 / K 2 CO 3 solution, add 1mL acetonitrile and stir, pass N 2 , heated to 85°C to dry the solvent, then repeatedly added 1 mL of acetonitrile and dried twice (3 times in total), to remove all K 18 water in F. Take 4mg of CMET-1 reactant, dissolve in 1mL of anhydrous DMSO, mix well and then N 2 Added into the reactor under protection, heated to 110°C for 30min. Purified by preparative HPLC 18 F-cMET-1. 1 H NMR (300MHz, CDCl 3)δ8.85(d,J=2.6Hz,1H),8.31(s,1H),8.24–8.10(m,1H),7.84–7.67(m,2H),7.57(t,J=7.9Hz,1H ),7.35(s,1H),6.66(s,1H),5.45–5.17(m,1H),4.96(d,J=6.1Hz,1...
Embodiment 2
[0064] 1) 68 Synthesis and characterization of Ga-NODAGA-cMET-1 probe;
[0065]
[0066] Add NODAGA-cMET-1 to the containing 68 In the ethanol solution of the Ga radioactive isotope, adjust the pH to an appropriate value, react for 30 minutes, and then use a C18 elution column to elute the product, and the radioactive detector detects that the labeling is successful.
[0067] 2) Glioma 68 Molecular imaging of Ga-NODAGA-cMET-1 in vivo
[0068] Establishment of nude mouse glioma (U87MG) subcutaneous xenograft tumor model, probe 68 One hour after Ga-NODAGA-cMET-1 injection, a 5-minute static PET imaging scan was performed, combined with a CT scan. The result is as Figure 4 . 68 Ga-NODAGA-cMET-1 was obviously aggregated in the tumor site 2 hours after intravenous injection, while the probe was not aggregated in the control group OVCAR3 tumor (negative expression of C-MET). After being blocked by a large amount of unlabeled NODAGA-cMET-1, the accumulation amount of the p...
Embodiment 3
[0072] 1) 18 Synthesis and characterization of F-cMET-2 probe;
[0073]
[0074] Reagent: 4 mg cMET-2, K 18 F, K 222 / K 2 CO 3 solution ( 18 For F labeling, containing 15mgK 222 and 3.5mgK 2 CO 3 per mL), dry DMSO.
[0075] Operation process:
[0076] use standard 18 F radioactive labeling reaction apparatus for labeling. Concretely, the K 18 F(K 2 CO 3 Neutralize H 18 F produced), add 1mLK 222 / K 2 CO 3 solution, add 1mL acetonitrile and stir, pass N 2 , heated to 85°C to dry the solvent, then repeatedly added 1 mL of acetonitrile and dried twice (3 times in total), to remove all K 18 water in F. Take 4mg of CMET-2 reactant, dissolve in 1mL of anhydrous DMSO, mix well and then N 2 Added into the reactor under protection, heated to 110°C for 30min. Purified by preparative HPLC 18 F-CMET-2. 1 H NMR (300MHz, Chloroform-d) δ8.78 (d, J=2.7Hz, 1H), 7.71(s, 1H), 7.24–7.21(m, 1H), 6.87(s, 1H), 5.35–5.19( m, 1H), 4.19(s, 2H), 3.83(d, J=28.6Hz, 4H), 3.30(d, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com