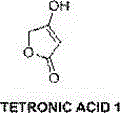
Novel synthesis technology of tetronic acid
A synthesis process, a technology for quaternary keto acid, applied in the field of brand-new synthesis technology of quaternary keto acid, can solve the problems of dangerous and complicated operation, high cost, long synthesis route, etc., and achieve a shortened reaction route, improved total yield and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A kind of brand-new synthetic technique of tetronic acid, specifically comprises the following steps
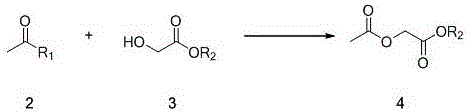
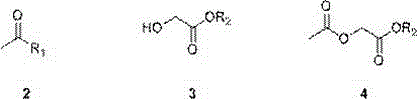
[0021] (a) Preparation of ethyl 2-acetoxyacetate
[0022] In a reactor equipped with magnetic stirring, at 0°C, dissolve 7.9g of acetyl chloride (0.1mol) in 30ml of tetrahydrofuran, add 10.6g (1.05 eq) of triethylamine, and then slowly add 10.4 g glycolic acid ester (0.1mol), dropwise completed, raised to 10 ℃, stirred for 1h, evaporated part of the solvent under reduced pressure, added an appropriate amount of water, extracted three times with tetrahydrofuran, combined the organic phases and dried, evaporated the organic solvent under reduced pressure to obtain 2-Acetoxyacetate (14.6 g g, 100% yield).
[0023] (b) Preparation of the target product tetronic acid
[0024] Dissolve the above 14.6g of 2-acetoxyacetate and 16.2g of potassium tert-butoxide in 146g of tetrahydrofuran, slowly raise the temperature to reflux (50°C), react for 2h, follow the completion of the...
Embodiment 2
[0028] (a) Preparation of ethyl 2-acetoxyacetate
[0029] In a reactor equipped with magnetic stirring, at 0°C, dissolve 12.3g of acetyl bromide (0.1mol) in 30ml of methyl tert-butyl ether, add 34.8g (1.05 eq) of triethylamine, and then Slowly add 10.4g of glycolic acid ester (0.1mol) dropwise under low temperature. After the dropwise addition, raise to 30°C, stir for 1h, evaporate part of the solvent under reduced pressure, add an appropriate amount of water, extract three times with tetrahydrofuran, combine the organic phases and dry them under reduced pressure. The organic solvent was evaporated to give 2-acetoxyacetate (14.6 g g, 100% yield).
[0030] (b) Preparation of the target product tetronic acid
[0031] Dissolve the above 14.6g of 2-acetoxy acetate and 48.6g of potassium tert-butoxide in 292g of methyl tert-butyl ether, slowly raise the temperature to reflux state (150°C), react for 12h, follow the complete reaction by HPLC, filter, The organic phase was discarde...
Embodiment 3
[0035] (a) Preparation of ethyl 2-acetoxyacetate
[0036] In a reactor equipped with magnetic stirring, at 0°C, dissolve 12.3g of acetyl bromide (0.1mol) in 30ml of tetrahydrofuran, add 23.2g (1.05 eq) of triethylamine, and then slowly add 10.4 g glycolic acid ester (0.1mol), dropwise completed, raised to 15 ℃, stirred for 1h, evaporated part of the solvent under reduced pressure, added an appropriate amount of water, extracted three times with tetrahydrofuran, combined the organic phases and dried, evaporated the organic solvent under reduced pressure to obtain 2-Acetoxyacetate. (14.6 g g, 100% yield).
[0037] (b) Preparation of the target product tetronic acid
[0038] Dissolve the above 14.6g of 2-acetoxyacetate and 32.4g of potassium tert-butoxide in 438g of tetrahydrofuran, slowly raise the temperature to reflux (75°C), react for 5h, follow the completion of the reaction by HPLC, filter, discard the organic phase, The obtained solid was washed three times with tetrahy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com