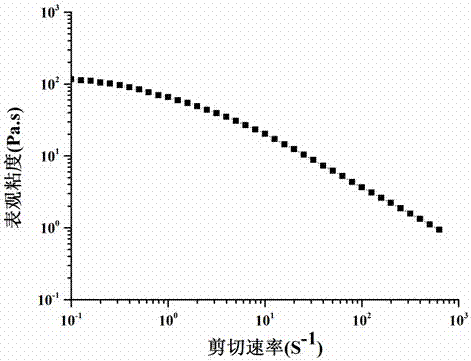
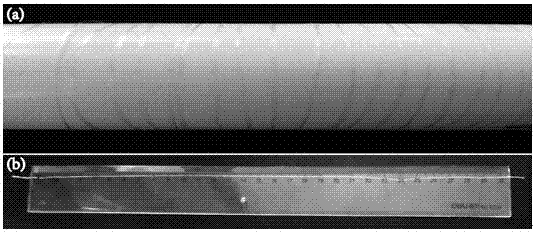
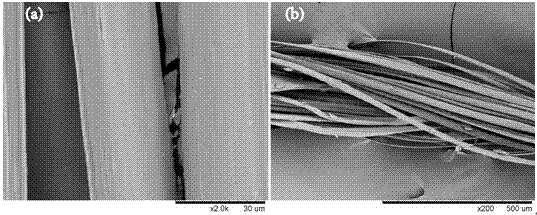
A composite drug-loaded fiber for surgical sutures
A technique for surgical suture and drug loading, which is applied in the directions of surgery, fibrous chemical characteristics, cellulose/protein conjugated rayon, etc. It can solve the problems of few sutures, easy deformation, easy infection, etc., and achieve good drug slow release. performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of Bacterial Cellulose A and Chitin B Solid Content A: (A+B) = 50% (w / w), Cellulose Microcrystalline C: (A+B) = 1% (w / w), Ciprosa Star D: (A+B)=1% (w / w) a composite drug-loaded fiber for surgical sutures and its preparation method:
[0032] Step (1): Solvent selection and dissolution of bacterial cellulose, chitin, cellulose microcrystals, and ciprofloxacin respectively.
[0033] Dissolve the bacterial cellulose with DMAc / LiCl mixed solvent system, place the bacterial cellulose in a freeze dryer at -50°C for 2 to 3 days, take it out and crush it, impregnate 2 g of freeze-dried bacterial cellulose in 98 g of 6 %(w / w) LiCl mixed solvent system of DMAc / LiCl, stirred and swelled at a speed of 50-100 rpm at room temperature, dissolved for 2 days until fully dissolved, and bacterial cellulose spinning with a solid content of 2% (w / w) was obtained. silk liquid;
[0034] Chitin was dissolved in DMAc / LiCl mixed solvent system, weighed 2 g chitin and placed in 98 g ...
Embodiment 2
[0041] Preparation of Bacterial Cellulose A and Chitin B Solid Content A: (A+B) = 10% (w / w), Cellulose Microcrystalline C: (A+B) = 2% (w / w), Ciprosa Star D: (A+B)=2% (w / w) a composite drug-loaded fiber for surgical sutures and its preparation method:
[0042] Step (1): Solvent selection and dissolution of bacterial cellulose, chitin, cellulose microcrystals, and ciprofloxacin respectively.
[0043] Weigh 3 g of freeze-dried bacterial cellulose and impregnate it in 97 g of DMAc / LiCl mixed solvent system containing 7% (w / w) LiCl, stir and swell at room temperature at a speed of 50-100 rpm, and dissolve for 2 days until fully dissolved. A bacterial cellulose spinning solution with a solid content of 3% was obtained; 3 g of chitin was weighed and placed in 97 g of DMAc / LiCl solvent containing 7% (w / w) LiCl, and the solid content was obtained according to the treatment method in Example 1. The content is 3% chitin spinning solution; the preparation of 10% cellulose microcrystallin...
Embodiment 3
[0047] Preparation of Bacterial Cellulose A and Chitin B Solid Content A: (A+B) = 30% (w / w), Cellulose Microcrystalline C: (A+B) = 1% (w / w), Ciprosa Star D: (A+B)=1% (w / w) a composite drug-loaded fiber for surgical sutures and its preparation method:
[0048] Step (1): Solvent selection and dissolution of bacterial cellulose, chitin, cellulose microcrystals, and ciprofloxacin respectively.
[0049] Weigh 2.5 g of freeze-dried bacterial cellulose and impregnate it in 97.5 g of DMAc / LiCl mixed solvent system containing 8% (w / w) LiCl, stir and swell at room temperature at a speed of 50-100 rpm, and dissolve for 2 days until fully dissolved. Obtain a bacterial cellulose spinning solution with a solid content of 2.5% (w / w); weigh 2.5 g of chitin and place it in 97.5 g of DMAc / LiCl solvent containing 8% (w / w) LiCl, according to Example 1 The treatment method was to obtain a chitin spinning solution with a solid content of 2.5%; the preparation of the 10% cellulose microcrystalline ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com