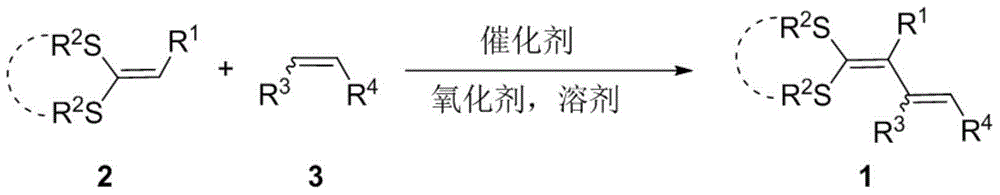
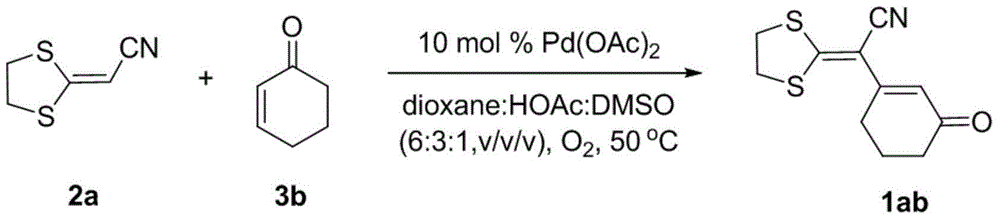Method for preparing 1,3-diolefins
A technology of diolefins and dithioketenes, which is applied in the field of preparation of 1,3-dienes, can solve the problems of high consumption of catalysts and oxidants, complex substrate synthesis, and harsh reaction conditions, so as to avoid pre-functionalization and product The effect of high yield and mild synthesis reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
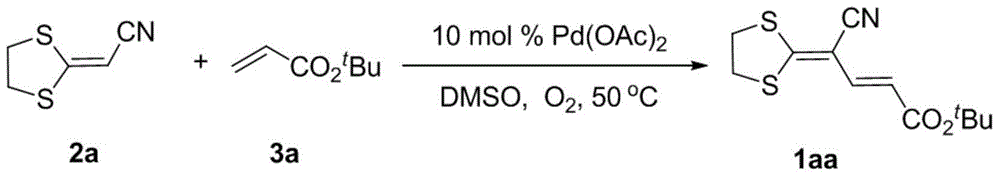
Embodiment 1
[0026]
[0027] Into a 10 mL Schlenk reaction tube, sequentially add palladium acetate (11 mg, 0.05 mmol), 2a (72 mg, 0.5 mmol), dimethyl sulfoxide (2 mL) and tert-butyl acrylate 3a (128 mg, 1.0 mmol) with oxygen as the oxidant (The reaction tube is connected to an oxygen balloon). The reaction was stirred at 50 °C for 24 h. After the reaction solution was cooled to room temperature, 20 mL of water was added, diatomaceous earth was used as a filter aid, and the filter cake (3×10 mL) was washed with ethyl acetate, and the organic phase was separated. Dry over anhydrous magnesium sulfate and filter. The volatile components were removed under reduced pressure, and then separated by silica gel column chromatography (eluent: petroleum ether (60-90°C) / ethyl acetate, v / v=5:1) to obtain the target product 1aa (16 mg, yield rate of 12%).
Embodiment 2
[0029] The reaction steps and operations are the same as in Example 1, except that the reaction solvent is a mixed solvent of acetic acid and dimethyl sulfoxide, and the volume ratio of the two is 3:1. The reaction was stopped, and the target product 1aa (29 mg, yield 22%) was obtained after post-processing. It shows that adding acetic acid can slightly increase the reaction yield.
Embodiment 3
[0031] The reaction steps and operations are the same as in Example 1, except that the reaction solvent is a mixed solvent of acetonitrile, acetic acid and dimethyl sulfoxide, and the volume ratio of the three is 6:3:1. The reaction was stopped, and the target product 1aa (95 mg, yield 72%) was obtained after post-processing. It shows that the ternary mixed solvent of acetonitrile, acetic acid and dimethyl sulfoxide is better than the binary mixed solvent of acetic acid and dimethyl sulfoxide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com