A kind of method adopting micro flow field reactor to prepare dimethylaminoethyl ginkgolide B
A technology of dimethylaminoethyl and dimethylaminoethyl chloride, which is applied in the field of medicine and can solve the problems of long reaction time, high reaction cost, and non-continuous preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
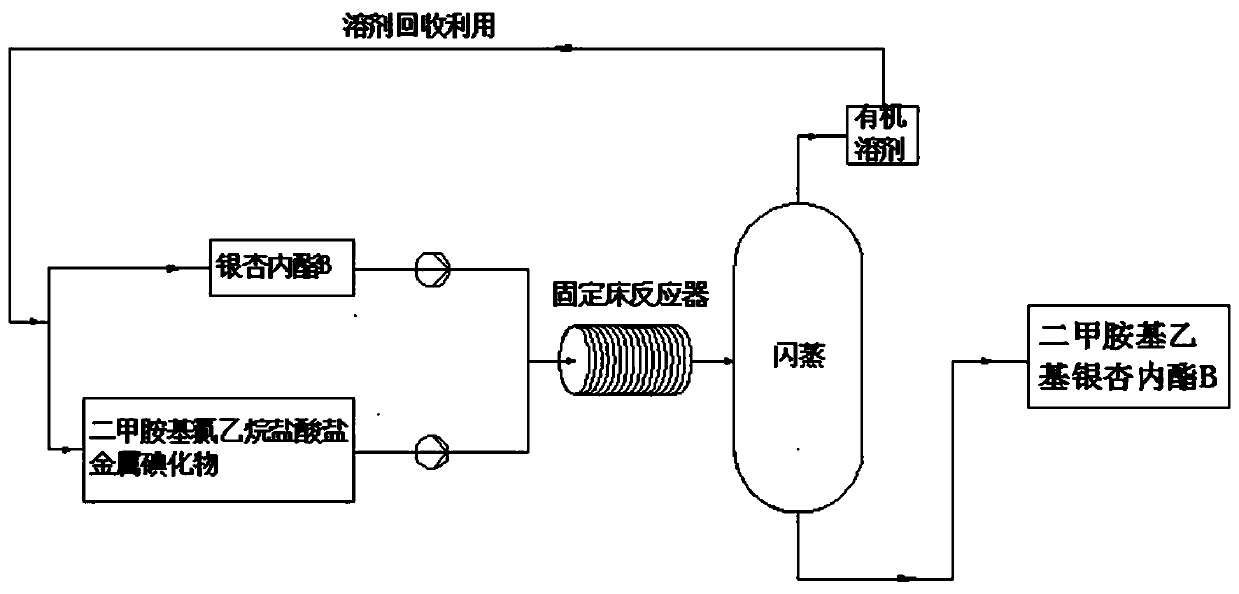
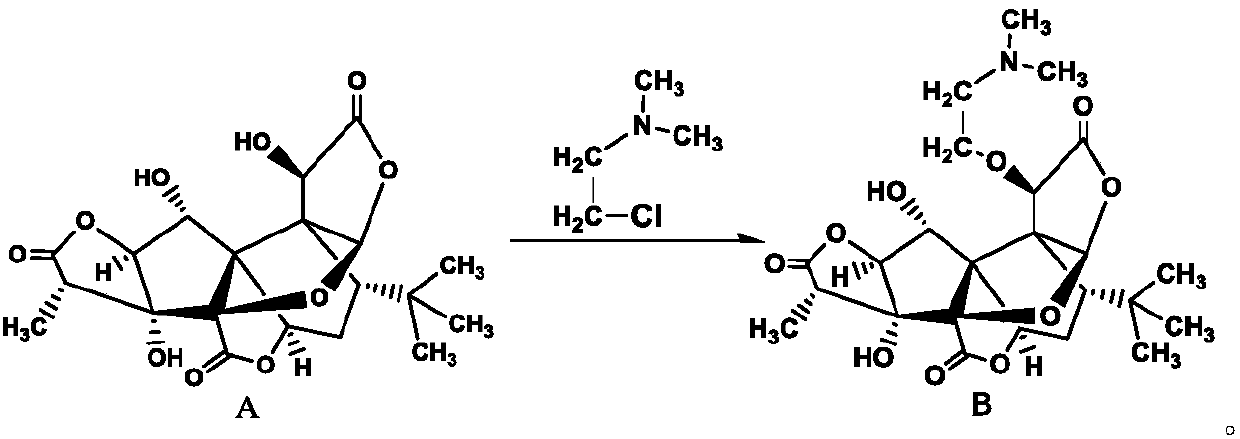
[0020] Dissolve dimethylaminoethyl chloride ethane hydrochloride and potassium iodide in the mixed solution of acetonitrile and water (the volume ratio of acetonitrile and water is 10%), then pump the acetonitrile solution of ginkgolide B and the above mixture into the In the fixed-bed microchannel modular reaction device of potassium carbonate, the mol ratio of ginkgolide B and dimethylaminoethyl chloride ethyl chloride hydrochloride is 1:1, and the mol ratio of potassium iodide and ginkgolide B is 0.2:1, The molar ratio of potassium carbonate to ginkgolide B is 2:1, the reaction residence time is kept for 4min, and the reaction is carried out at 70°C, the crude product is filtered, the filtrate is concentrated, and the obtained solid is recrystallized with methanol to obtain dimethylaminoethyl Ginkgolide B, the yield was 75%.
Embodiment 2
[0022] Dissolve dimethylaminoethyl chloride ethane hydrochloride and potassium iodide in the mixed solution of tetrahydrofuran and water (the volume ratio of tetrahydrofuran and water is 10%), then pump the tetrahydrofuran solution of ginkgolide B and the above mixture into the In the fixed-bed microchannel modular reaction device of cesium carbonate, the mol ratio of ginkgolide B and dimethylaminoethyl chloride ethyl chloride hydrochloride is 1:1, and the mol ratio of potassium iodide and ginkgolide B is 0.2:1, The molar ratio of cesium carbonate to ginkgolide B is 2:1, the reaction residence time is kept for 4min, and the reaction is carried out at 70°C, the crude product is filtered, the filtrate is concentrated, and the obtained solid is recrystallized with methanol to obtain dimethylaminoethyl Ginkgolide B, the yield was 71%.
Embodiment 3
[0024] Dissolve dimethylaminoethyl chloride hydrochloride and sodium iodide in a mixture of acetone and water (the volume ratio of acetone and water is 10%), then pump the acetone solution of ginkgolide B and the above mixture into In the fixed-bed microchannel modular reaction device that is filled with cesium carbonate, the mol ratio of ginkgolide B and dimethylaminoethyl chloride ethane hydrochloride is 1:1, and the mol ratio of sodium iodide and ginkgolide B is 0.2:1, the molar ratio of cesium carbonate to ginkgolide B is 2:1, keep the reaction residence time for 5min, react at 70°C, filter the crude product, concentrate the filtrate, and recrystallize the obtained solid with methanol to obtain dimethyl Aminoethyl ginkgolide B, the yield was 73%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com