Oral pharmaceutical composition for the treatment of albinism
A composition and albinism technology, applied in the field of medicine, can solve the problems of lack of effective treatment drugs for albinism, failure to find out, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
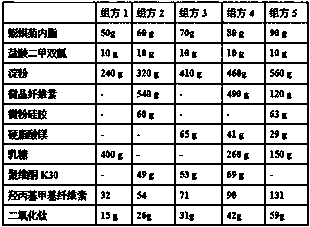
[0016] Example 1 Oral tablet for the treatment of albinism and its preparation
[0017] The oral tablet formulations used to treat albinism are as follows:
[0018]
[0019] Oral tablets for the treatment of albinism are prepared as follows:
[0020] 1) Take the prescription amount of wedelolactone and metformin hydrochloride and grind them separately, pass through an 80-mesh sieve, and mix to obtain raw material powder;
[0021] 2) Take the prescribed amount of starch and add water to prepare a starch slurry with a concentration of 5%;
[0022] 3) Add the raw material powder obtained in step 1) to the starch slurry obtained in step 2), then add the excipients in the prescription except hydroxypropyl methylcellulose and titanium dioxide, mix evenly, and press the tablet core;
[0023] 4) Add hydroxypropyl methylcellulose and titanium dioxide to ethanol to dissolve and disperse as a coating solution to coat the tablet cores in step 3).
Embodiment 2
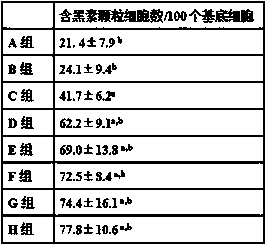
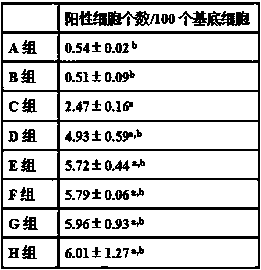
[0024] Example 2 Anti-albinism pharmacodynamics research and safety research
[0025] 144 male albino rats, 28 weeks old, weighing 320-350 g, were randomly divided into 8 groups, 18 rats in each group. During the experiment, free access to food and water, 12 hours of light and dark cycle light.
[0026] Group A was given normal saline by intragastric administration;
[0027] Group B was given metformin hydrochloride aqueous solution by intragastric administration, and the dose of metformin hydrochloride was 10 mg / kg;
[0028] Group C was given wedelia lactone aqueous suspension by intragastric administration, and the dose of wedelia lactone was 90 mg / kg;
[0029] Group D was given metformin hydrochloride and wedelolactin aqueous suspension by intragastric administration, and the doses of metformin hydrochloride and wedelia lactone were 10 mg / kg and 50 mg / kg respectively;
[0030] Group E was intragastrically given metformin hydrochloride and wedelide aqueous suspension, the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com