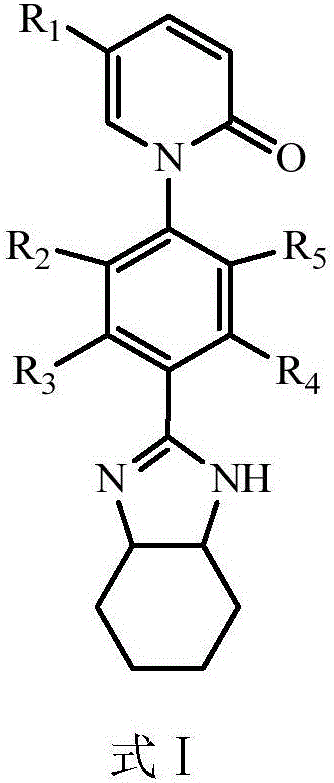
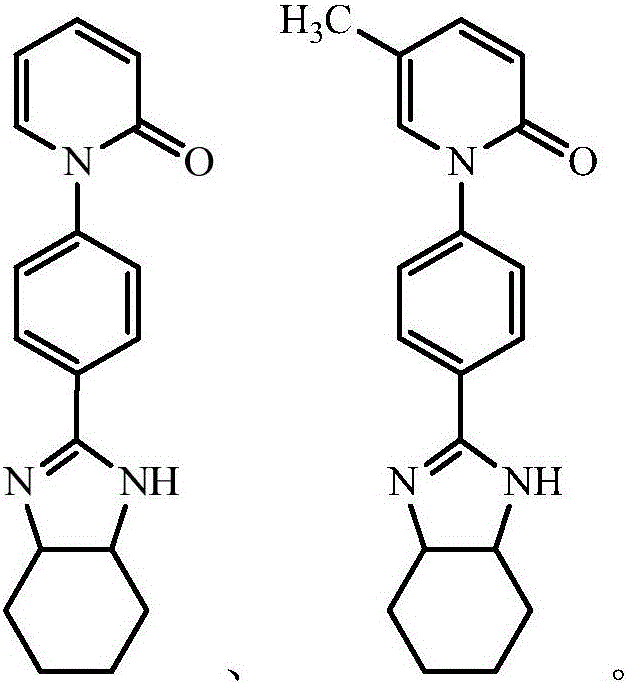
Pirfenidone derivative and preparation method thereof
A compound and hydrate technology, applied in the field of pirfenidone derivatives and its preparation, can solve the problems of poor anti-fibrosis activity and achieve good industrialization prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
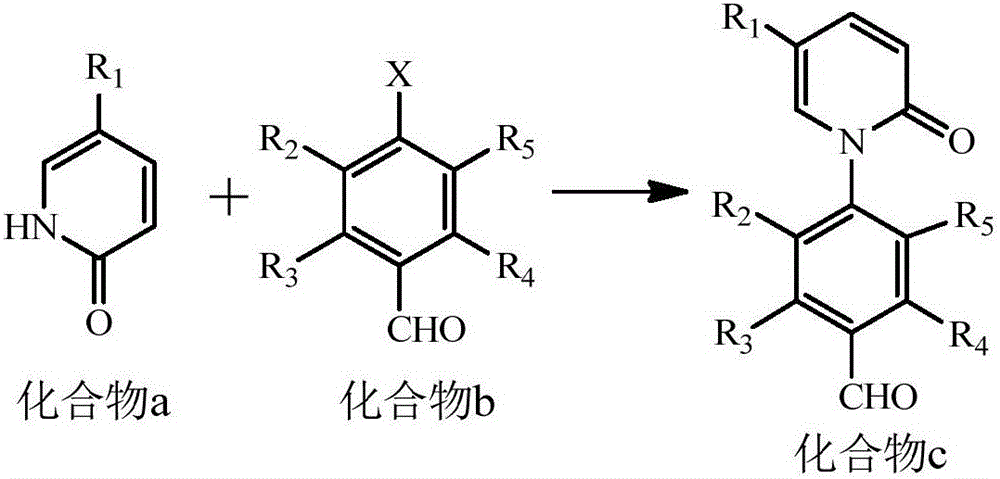
[0043] Example 1. Synthesis of compound 5e of the present invention
[0044] synthetic route:
[0045]
[0046] 1. Synthesis of compound 3 (5-methyl-2-(1H)-pyridone)
[0047] Add 3.40mL of 50% sulfuric acid (v / v) to the 25mL reaction flask, then add 1.00g (10mmol) of 2-amino-5-methylpyridine (compound 1), cool to below 10℃ in an ice salt bath, and stir for a while Minutes later, the reaction solution turned milky white; then slowly drop 1.72g (25mmol) NaNO 2 With 3mL H 2 The mixed solution composed of O, brownish-yellow gas with pungent odor is generated during the dropping process. After the addition, the reaction liquid turns to light yellow. Adjust the pH to 7-8 with 10% dilute sulfuric acid, reflux and stir the reaction for about 20 minutes. Remove most of the water, add an appropriate amount of 300 mesh silica gel to it, spin dry, pour it into a glass sand core funnel, rinse with ethyl acetate and filter with suction, spin the filtrate to dryness to obtain the crude product (co...
Embodiment 2
[0056] Example 2. Synthesis of compound 7a of the present invention
[0057] synthetic route:
[0058]
[0059] Using compound 1'(2-aminopyridine) as a raw material, according to a method similar to Example 1, compound 7a was prepared, and the single-step yield of the third step was 64%.
[0060] Compound 7a: 1-(4-(3a,4,5,6,7,7a-hexahydro-1H-benzo[d]imidazol-2-yl)phenyl)pyridine-2(1H)-one, yellow Solid, mp254-256℃;
[0061] 1 H NMR(400MHz,DMSO)δ8.07(d,J=8.6Hz,2H),7.83-7.69(m,3H),7.59-7.52(m,1H),6.51(d,J=9.3Hz,1H) ,6.38(td,J=6.7,1.1Hz,1H),3.50–3.37(m,1H),3.08(dd,J=14.5,7.2Hz,1H), 2.57(dd,J=11.1,6.1Hz,4H ),1.75(s,1H),1.44(dd,J=16.8,11.7Hz,4H);
[0062] 13 C NMR (101MHz, DMSO) δ163.61,160.91,145.35,141.14,138.39,129.26,127.76,122.32,120.64,106.21,56.01,53.84,31.94,25.33,23.97,18.45;
[0063] HRMS(ESI)calcd for C 18 H 19 N 3 O[M+H] + 294.1607, found 294.1604.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com