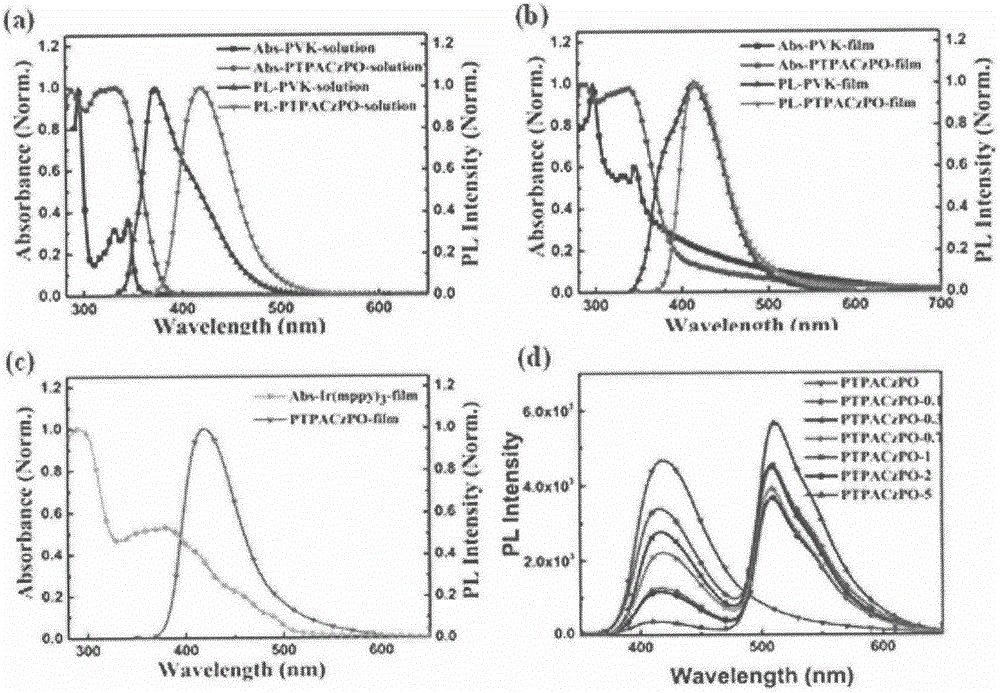
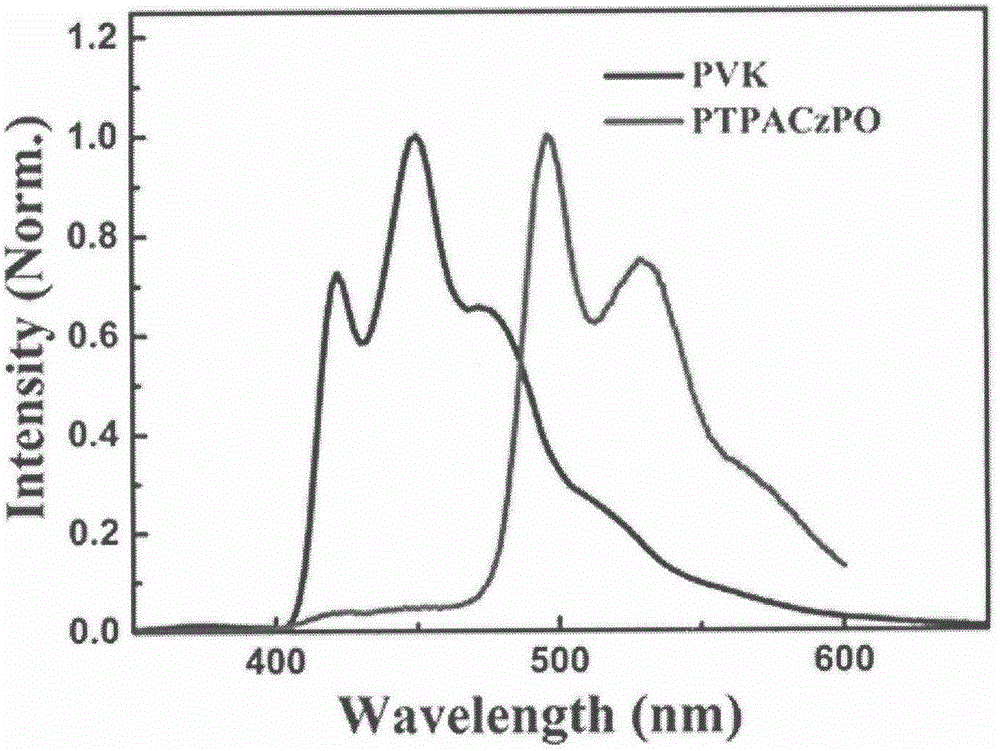
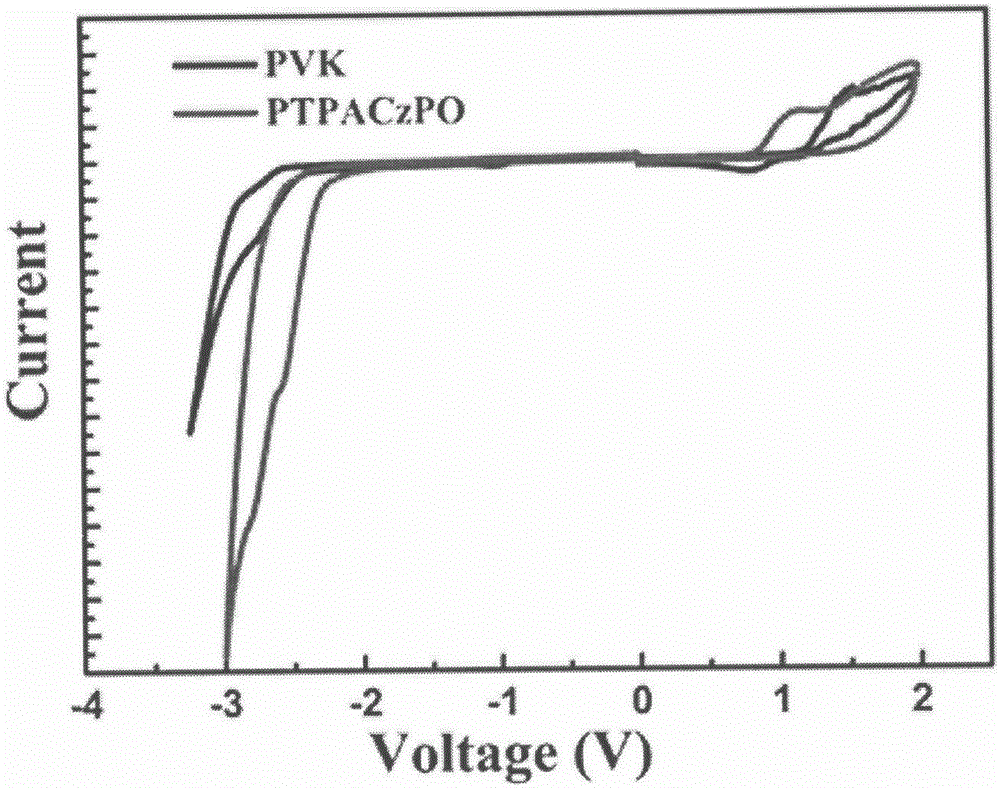
Vinyl polymer main body material with electron donor-acceptor structure and preparation and application methods therefor
A technology of main material and specific structure, which is applied in chemical instruments and methods, compounds of group 5/15 elements of the periodic table, luminescent materials, etc., can solve problems such as energy loss, and achieve excellent thermodynamic and morphological stability effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0040] Example 1: Synthesis of polymer material PTPACzPO precursor compound
[0041] Compound 3, the synthesis of 6-dibromocarbazole (2):
[0042] Under ice-water bath conditions, a solution of N-N-dimethylformamide (DMF) (20ml) dissolved with carbazole (10g, 59.8mmol) was added dropwise with a constant pressure dropping funnel to dissolve N-bromosuccinyl imine (21.28g, 120mmol) in DMF (30ml). After the dropwise addition was completed, the ice-water bath was removed, and the reaction was carried out overnight at room temperature and protected from light. Then, the reaction solution was washed with deionized water, and the solid was filtered out after precipitation, and recrystallized with ethanol to obtain product 2. Yield: 47%. 1 H NMR (400MHz, Chloroform-d) δ8.13(d, J=1.9Hz, 3H), 7.52(dd, J=8.6, 1.9Hz, 2H), 7.31(d, J=8.6Hz, 2H). 13 C NMR (75MHz, Chloroform-d) δ129.34, 123.28, 112.22.
[0043] Synthesis of compound 3-(diphenylphosphoryloxy)-6-bromocarbazole (4):
[0044...
example 2
[0065] Example 2: Synthesis of Polymer Material PVPPOK Precursor Compound
[0066] Synthesis of compound 3-bromocarbazole (2):
[0067] Under ice-water bath conditions, a solution of N-N-dimethylformamide (DMF) (20ml) dissolved in N-bromosuccinimide (10.64g, 59.8mol) was added dropwise to the solution with a constant pressure dropping funnel. In a solution of carbazole (10 g, 59.8 mol) in DMF (20 ml). After the dropwise addition was completed, the ice-water bath was removed, and the reaction was carried out overnight at room temperature and protected from light. Then, the reaction liquid was washed with deionized water, and the solid was filtered out after the solid was precipitated, and the product 2 was obtained by recrystallization from ethanol. Yield: 47%. 1 H-NMR (400MHz, CDCl3) δ8.19 (d, J = 1.9Hz, 1H), 8.09 (d, J = 6.9Hz, 1H), 8.05-8.01 (m, 1H), 7.50 (dd, J = 8.6 , 1.9Hz, 1H), 7.46-7.43(m, 2H), 7.31(d, J=8.6Hz, 1H), 7.28-7.22(m, 1H). 13 C-NMR (75MHz, CDC13) δ128.55...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com