Application of calcein and oseltamivir in drug for preventing and treating H7N9 influenza
A calcein, H7N9 technology, applied in the direction of medical preparations containing active ingredients, pharmaceutical formulations, organic active ingredients, etc., can solve the problem of no anti-influenza virus and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] H7N9 wild-type or mutant neuraminidase solution was prepared with pH 6.5 MES buffer (32.5mM), a certain amount of neuraminidase was added to the reaction buffer to make the total volume 40 μL, and then 10 μL of different concentration of calcein or oseltamivir carboxylate. Place in a 37°C incubator for 30 minutes, then add 50 μL of 20 μM MU-NANA substrate buffer solution, the final reaction system is 100 μL, the reaction solution is placed in a black microplate plate, and detected on a microplate reader. The excitation wavelength is 360 nm, and the emission wavelength is 360 nm. The wavelength is 450nm, and the detection time is 8min. The change of the detected fluorescence intensity represents the activity of the enzyme to degrade the substrate. The test results are: IC of oseltamivir carboxylate on H7N9 wild-type and mutant neuraminidase 50 1.75±0.12nmol / L and 26.52±1.06μmol / L, respectively; the IC of calcein on H7N9 wild-type and mutant 50 They were 12.68±2.26μm / L...
Embodiment 2
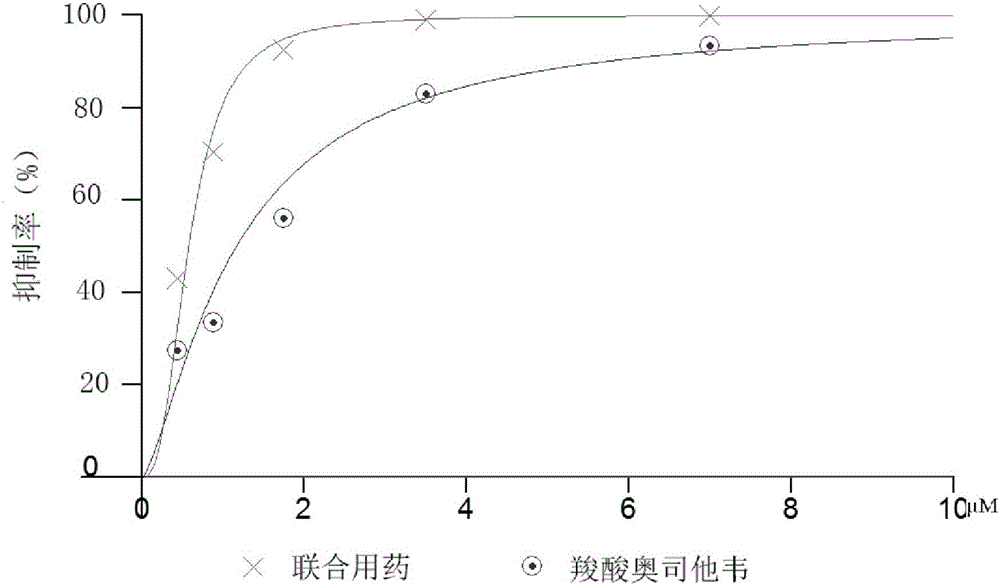
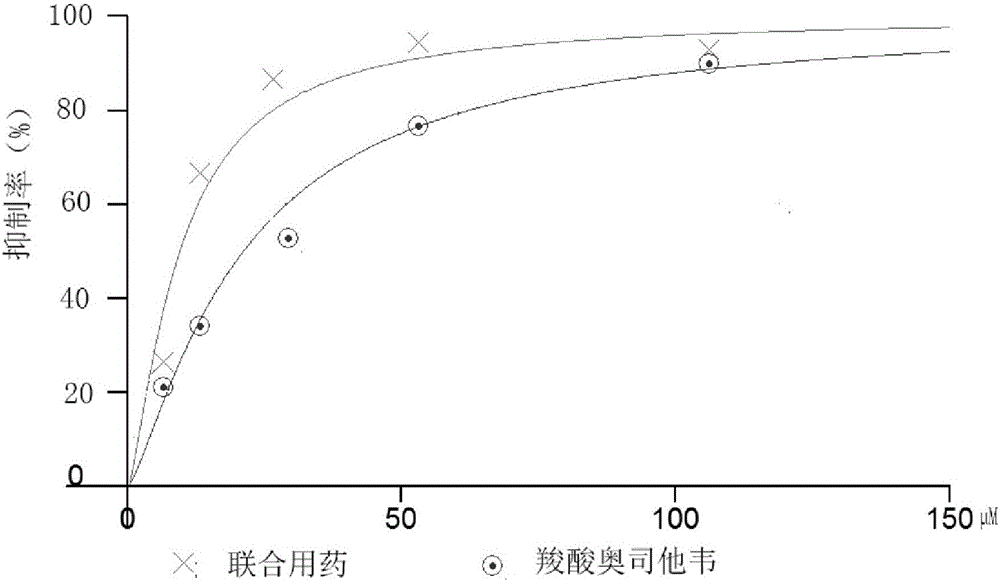
[0016] According to the determined IC of calcein and oseltamivir carboxylate on H7N9 wild-type and mutant neuraminidase 50 To determine the adding concentration of calcein and oseltamivir carboxylate. The concentrations of calcein and oseltamivir carboxylate were chosen to be 0.25, 0.5, 1, 2 and 4 times the IC of H7N9 wild-type and mutant neuraminidase, respectively 50 concentration. And the ratio of the concentration of calcein added to the concentration of oseltamivir carboxylate is maintained consistent, its IC 50 value ratio. First, add 30 μL of diluted H7N9 wild-type or mutant neuraminidase solution to a 96-well ELISA plate, and then add 10 μl of calcein of different concentrations and 10 μL of corresponding concentrations of oseltamivir carboxylate to each well , each concentration was repeated 4 times. After incubating at 37°C for 30 min, 50 μl of 20 μM NA-specific substrate MU-NANA was added to detect the fluorescence intensity of H7N9 wild-type and mutant neuramin...
Embodiment 3
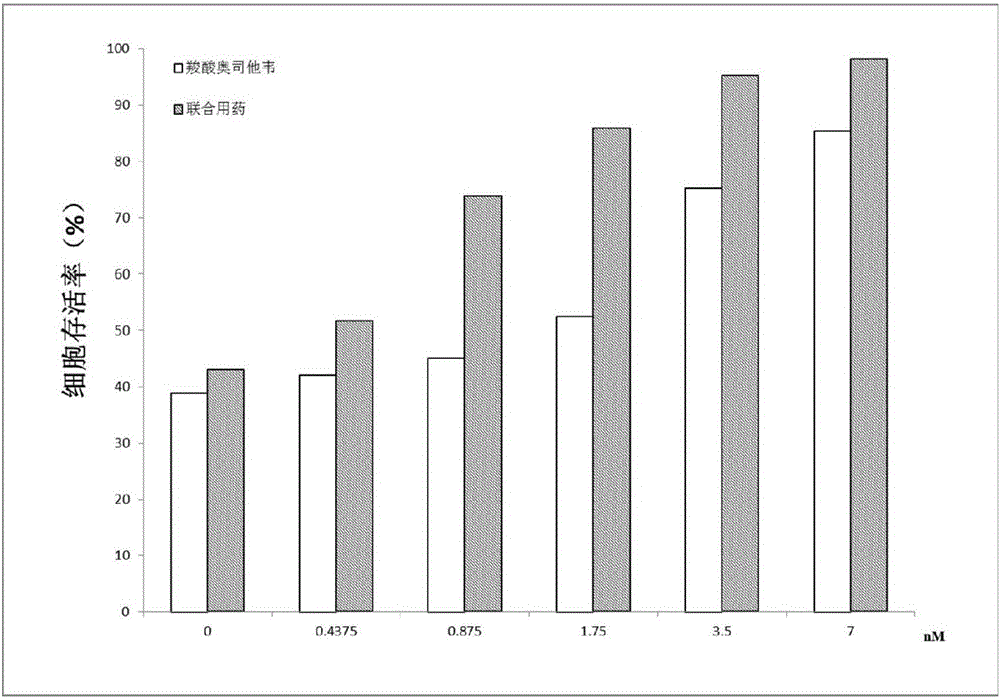
[0021] In order to detect the effect of the mixture of calcein and oseltamivir carboxylate on MDCK cells against H7N9 wild-type and mutant influenza viruses, MDCK cells in logarithmic growth phase were selected. Spread 96-well plates, 3000 / well, 37°C, 5% CO 2 Cultivate in an incubator for 24 hours until the confluence of monolayer cells reaches 50-60%. The next day, the culture medium was discarded and washed twice with serum-free DMEM to remove residual serum. Then use 100 μL of H7N9 wild-type or mutant influenza virus allantoic fluid (100-fold concentration of TCID 50 concentration) and 100 μl of mixtures of calcein and oseltamivir carboxylate at different concentrations were mixed for 2 hours at 37°C. IC50 concentrations of 0.25, 0.5, 1, 2 and 4 times, the ratio of the concentration of calcein added to the concentration of oseltamivir carboxylate remained consistent, and its IC 50 value ratio. After that, the incubation mixture was added to the 96 wells where the cells ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com