A kind of pharmaceutical composition and application thereof for the treatment of hyperuricemia
A technology for hyperuricemia and a composition, applied in the field of medicine, can solve problems such as large side effects and poor treatment effect, and achieve the effects of improving medication safety and reducing medication risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
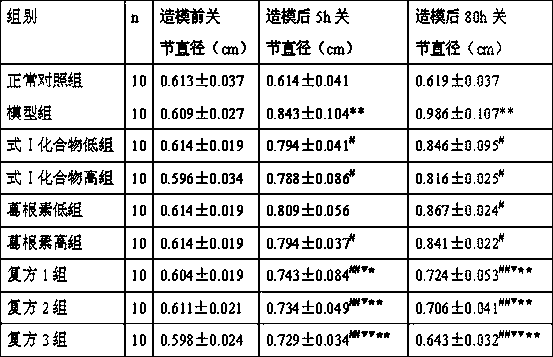
Image
Examples
Embodiment 1
[0020] The preparation of embodiment 1 compound tablet
[0021] Puerarin
[0022] Preparation process: mix puerarin and the compound of formula I with auxiliary materials microcrystalline cellulose and starch evenly, add appropriate amount of 15% starch slurry to make soft material, and then granulate through a 16-mesh sieve. Dry the wet granules at 60°C, pass the dry granules through a 16-mesh sieve for granulation, sieve out the fine powder in the dry granules, mix with magnesium stearate, and then mix with the dry granules, and press into tablets to obtain the product.
Embodiment 2
[0023] The preparation of embodiment 2 compound tablet
[0024] Puerarin
[0025] Preparation process: except that the components are different, the preparation process is the same as that described in Example 1.
Embodiment 3
[0026] The preparation of embodiment 3 compound dispersible tablets
[0027] Puerarin
[0028] Preparation process: Weigh puerarin and the compound of formula I according to the prescription, use microcrystalline cellulose as filler, croscarmellose sodium and polyvinylpyrrolidone as disintegrants, and 5% PVP 60% ethanol solution as adhesive Agent, micronized silica gel as a flow aid, granulated in one step in a fluidized bed, and then compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com