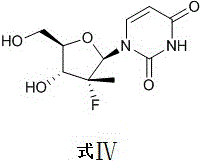
Preparation method of (2'R)-2'-deoxidation-2'-fluorine-2'-methylurea glucoside
A technology of methyl uridine and methyl, applied in the field of preparation of (2'R)-2'-deoxy-2'-fluorine-2'-methyl uridine, can solve the problem of unsuitable large-scale industrial production, technology Complex routes and other problems, to achieve the effect of reducing the amount of solvent used, the overall yield is high, and reducing the generation of solid waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
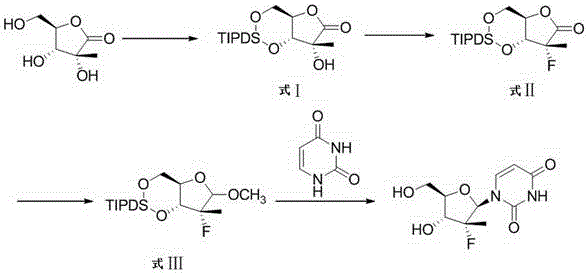
[0026] 1. Preparation of intermediate Ⅰ
[0027] Under anhydrous and oxygen-free conditions, add 80g 2-C-methyl-D ribono-1,4-lactone, 4g 4-dimethylaminopyridine, 187g 1,3-dichloro-1, 1,3,3-Tetraisopropyl dimethylsiloxane and 1500mL acetonitrile, stirred, cooled to 15-20°C to react, TLC to monitor the reaction; after the reaction was completed, evaporate acetonitrile under reduced pressure, add 1000mL ethyl acetate and 300mL saturated Sodium bicarbonate solution, stirred, left to stand for liquid separation, the aqueous layer was extracted with ethyl acetate (800mL×2), the organic phases were combined, washed with sodium chloride solution, dried over anhydrous sodium sulfate, filtered with suction, concentrated to dryness under reduced pressure , to obtain crude brown-yellow solid. The crude product was refined with 5 times the amount of isopropanol to obtain 169 g of light yellow solid product. Yield: 85%.
[0028] 2. Preparation of Intermediate II
[0029] Add 169g of int...
Embodiment 2
[0037] 1. Preparation of intermediate Ⅰ
[0038] Under anhydrous and oxygen-free conditions, add 65g 2-C-methyl-D ribono-1,4-lactone, 8g N,N-diisopropylethylamine, 150g 1,3-di Chloro-1,1,3,3-tetraisopropyl dimethyl siloxy ether and 1000mL tetrahydrofuran, stirred, cooled to 15-20°C to react, TLC to monitor the reaction; after the reaction was completed, distill off tetrahydrofuran under reduced pressure, add 600mL toluene and 100mL saturated sodium bicarbonate solution, stirred, allowed to stand for liquid separation, extracted the aqueous layer with toluene (400mL×2), combined the organic phases, washed with sodium chloride solution, dried over anhydrous sodium sulfate, suction filtered, and concentrated to dryness under reduced pressure , to obtain crude brown-yellow solid. The crude product was refined with 5 times the amount of isopropanol to obtain 137 g of light yellow solid product. Yield: 86%.
[0039] 2. Preparation of Intermediate II
[0040] Add 137g of intermed...
Embodiment 3
[0048] 1. Preparation of intermediate Ⅰ
[0049] Under anhydrous and oxygen-free conditions, add 48g 2-C-methyl-D ribono-1,4-lactone, 8g piperidine, 112g 1,3-dichloro-1,1,3,3 -Tetraisopropyl dimethyl siloxy ether and 800mL acetonitrile, stirred, cooled to 15-20°C to react, TLC to monitor the reaction; after the reaction was completed, the acetonitrile was evaporated under reduced pressure, and 800mL ethyl acetate and 200mL saturated sodium bicarbonate solution were added, Stir, stand still for liquid separation, extract the aqueous layer with ethyl acetate (300mL×2), combine the organic phases, wash with sodium chloride solution, dry over anhydrous sodium sulfate, filter with suction, and concentrate to dryness under reduced pressure to obtain a brown-yellow solid Crude. The crude product was refined with 5 times the amount of isopropanol to obtain 101 g of a light yellow solid product. Yield: 85%.
[0050] 2. Preparation of Intermediate II
[0051] Add 101g of intermediat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com