Angiotensin-converting enzyme inhibitory peptide and its preparation method and application
An angiotensin and inhibitory peptide technology, applied in the field of biomedicine, can solve problems such as elevated blood pressure, and achieve the effects of strong operability, good application prospects, and reasonable process design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A method for preparing an angiotensin-converting enzyme inhibitory peptide, comprising the following steps:
[0040] (1) Preparation of variegated clam enzymatic hydrolyzate:
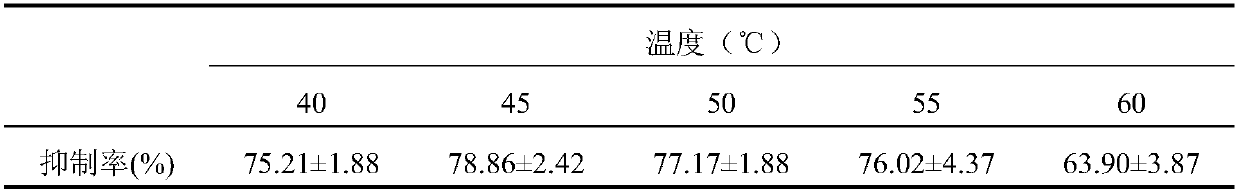
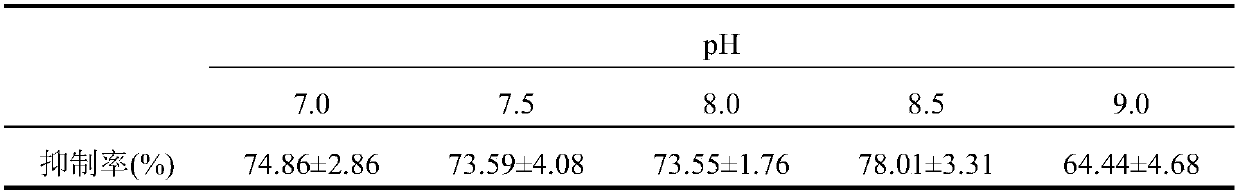
[0041] Wash the variegated clam soft and silt, add 3 times the amount of water to decoct twice, 40 minutes each time, separate the decoction and meat dregs, drain; take the meat dregs, add 3 times the amount of water to homogenize, add enzyme activity 30000U / g of trypsin enzymolysis, the weight of trypsin added is 0.60% of the weight of meat dregs, the temperature of enzymolysis is 45°C, the pH of enzymolysis is 8.50, and the reaction time of enzymolysis is 2h; after the end of enzymolysis, Inactivate in a boiling water bath, then add a certain amount of absolute ethanol for alcohol precipitation, and finally form a 70% alcohol solution, and let it stand at 4°C for 24 hours, then take the supernatant, concentrate, and freeze-dry to obtain freeze-dried pink;
[0042] (2) Purification by ion exch...
Embodiment 2
[0046] Example 2 Sequence Analysis of Angiotensin Converting Enzyme Inhibitor Peptide
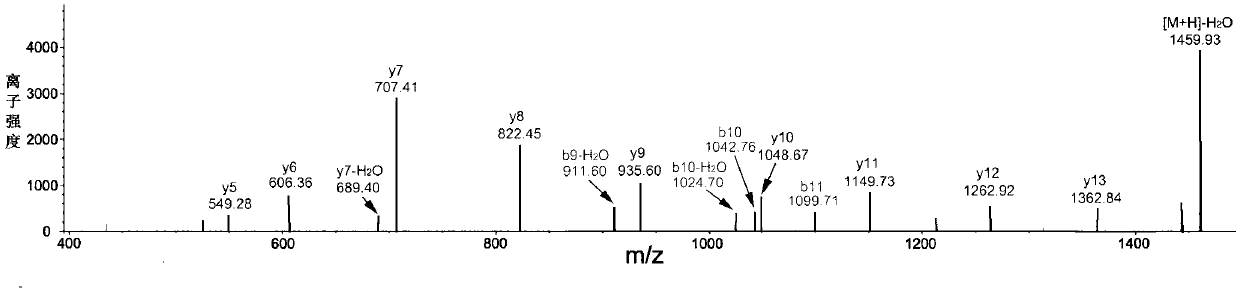
[0047] Take the angiotensin-converting enzyme inhibitory peptide prepared in Example 1, and use Nano-LC-ESI-MS / MS to analyze the amino acid sequence of the polypeptide. The mass spectrometry conditions are: ESI source, scanning mode: positive ion mode, mass scanning range: 50 ~1000m / z; The molecular weight of the active polypeptide obtained by analysis is 1477.80Da, and the mass spectrometry detection picture is as follows figure 1 As shown, the determined amino acid sequence is: Asn-Thr-Leu-Thr-Leu-Ile-Asp-Thr-Gly-Ile-Gly-Met-Thr-Lys.
Embodiment 3
[0048] Example 3 Synthesis of angiotensin-converting enzyme inhibitory peptide
[0049] According to the amino acid sequence obtained in Example 2, the angiotensin-converting enzyme inhibitory peptide Asn-Thr-Leu-Thr-Leu-Ile-Asp-Thr-Gly-Ile-Gly-Met-Thr- Lys, the purity of the synthesized polypeptide was 98% by HPLC analysis, the molecular weight determined by mass spectrometry was 1477.80Da, which was consistent with the molecular weight of the purified polypeptide, and the fragments of the secondary mass spectrum were consistent with the purified polypeptide fragments.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com