Dihydric alcohol containing acylhydrazone bond, dihydric alcohol containing acylhydrazone bond and disulfide bond, self-repairing polyurethane elastomer and preparation method thereof
A technology of polyurethane elastomer and polyurethane prepolymer is applied in the field of self-healing polyurethane elastomer and its preparation, and achieves the effects of simple preparation method, strong performance controllability and high self-healing efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The preparation method of the dihydric alcohol containing acylhydrazone bond comprises the following steps: dissolving 20-30 parts by weight of hydroxyaldehyde in isopropanol, dissolving 10-20 parts by weight of dihydrazide compound in ice In the acetic acid, the isopropanol and glacial acetic acid are mixed in a weight part of 120-150 parts by weight, and reacted at 50°C-70°C to obtain a dihydric alcohol containing an acylhydrazone bond. In the mixed solution of isopropanol and glacial acetic acid, isopropanol: glacial acetic acid (weight ratio) = 1:2-1:5.
[0032] The present invention also provides dihydric alcohols containing acylhydrazone bonds and disulfide bonds, which are obtained by reacting the following raw materials in parts by weight: 20-40 parts of dithiolipid monomers, 40-60 parts of hydrazine hydrate and 20- 30 parts of hydroxy aldehydes, in parts by weight.
[0033] The dithiolipid monomers are 3,3'-dithiodipropionic acid dimethyl ester, 2,2'-dithiodia...
Embodiment 1
[0040] Embodiment 1: Preparation contains the dibasic alcohol of acylhydrazone bond
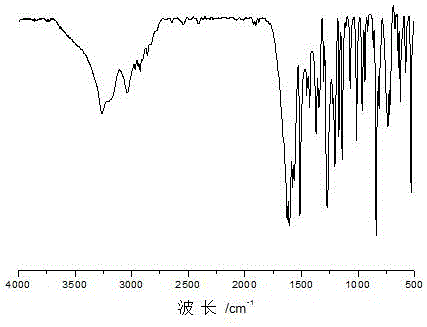
[0041] Dissolve 24.4g of p-hydroxybenzaldehyde (0.2mol-CHO) in 48.8g of isopropanol, and dissolve 17.4g of adipic acid dihydrazide (0.2mol of hydrazide group) in 87.0g of glacial acetic acid; mix the two The solution was mixed evenly and reacted at 70°C for 1 h under stirring at 250 rpm. Filtration under reduced pressure, the obtained product was washed with 150g ethanol (3 times, 50g each time) and 600g deionized water (3 times, 200g each time) successively under heating at 70°C, and dried to obtain a dihydrazone bond-containing Alcohol, that is, p-hydroxybenzaldehyde adipyl hydrazone. The product is light yellow solid powder. The productive rate is 96%, and its infrared spectrogram is as follows figure 1 As shown, the molecular structural formula of the product obtained is as follows:
[0042] .
Embodiment 2
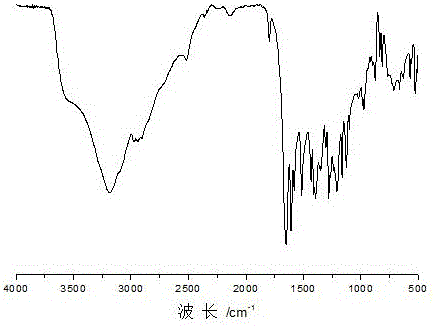
[0043] Embodiment 2: Preparation contains the dibasic alcohol of acylhydrazone bond and disulfide bond
[0044] 1. Preparation of 3,3'-dithiobis(propionohydrazide)
[0045] Take 29.90 g of dimethyl 3,3'-dithiodipropionate and dissolve it in 60 g of methanol, then add 60 g of hydrazine hydrate, and stir at room temperature for 1 h. The product was filtered with quantitative filter paper under reduced pressure, and the obtained product was washed with 100 g of methanol, 100 g of deionized water, and 100 g of methanol in sequence, filtered, and dried to obtain 24.68 g of 3,3'-dithiobis(propionohydrazide). Yield 86%. The product is a white solid powder, and the molecular structural formula of 3,3'-dithiobis(propionohydrazide) is as follows:
[0046] .
[0047] 2. Preparation of dihydric alcohols containing acylhydrazone bonds and disulfide bonds
[0048] Get p-Hydroxybenzaldehyde 24.4g and dissolve in 48.8g isopropanol, get 3,3'-dithiobis(propionohydrazide) 23.8g that embodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com