Solid electrolyte material and preparation method thereof
A solid electrolyte and raw material technology, applied in the direction of solid electrolyte, electrolyte, non-aqueous electrolyte, etc., can solve problems such as explosive, flammable, and liquid leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
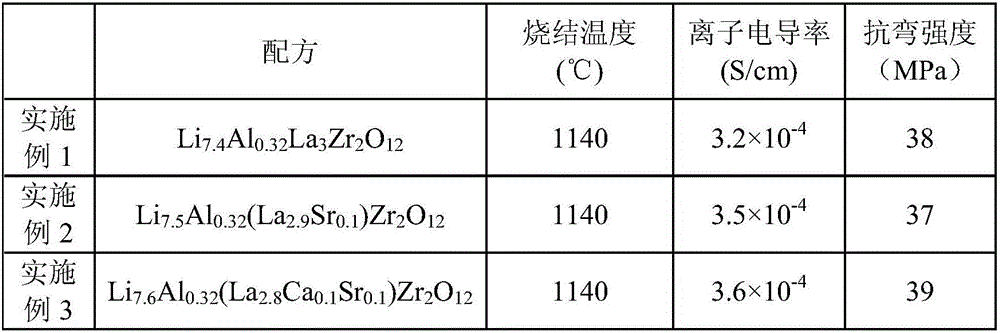
Embodiment 1-3
[0054] According to the general formula of chemical composition Li 6+x Al y (La z A 3-z )Zr 2 o 12 Ingredients, wherein A is at least one selected from Ca, Sr, Y, and Ba, and satisfies 0≤x≤2, 0<y≤1, 2≤z≤3, and the solid of this embodiment is prepared through the following steps Electrolyte material:
[0055] (1) Using analytically pure chemical reagent Li 2 CO 3 、Al 2 o 3, La 2 o 3 , CaCO 3 、BaCO 3 , ZrO 2 , SrCO 3 , Y 2 o 3 as raw material, according to Li 6+x Al y (La z A 3-z )Zr 2 o 12 The molar ratio of the general chemical formula, wherein 0≤x≤2, 0<y≤1, 2≤z≤3. Put the prepared raw materials into a 50KG horizontal ceramic ball mill for ball milling for 16 hours;
[0056] (2) After drying the ball-milled slurry, press it into a large sheet with a diameter of 40 mm under a pressure of 30 MPa, and pre-burn it at 900 ° C for 4 hours at a heating rate of 4 ° C / min to obtain a sintered block;
[0057] (3) Cross 60 mesh sieves after the burnt block afte...
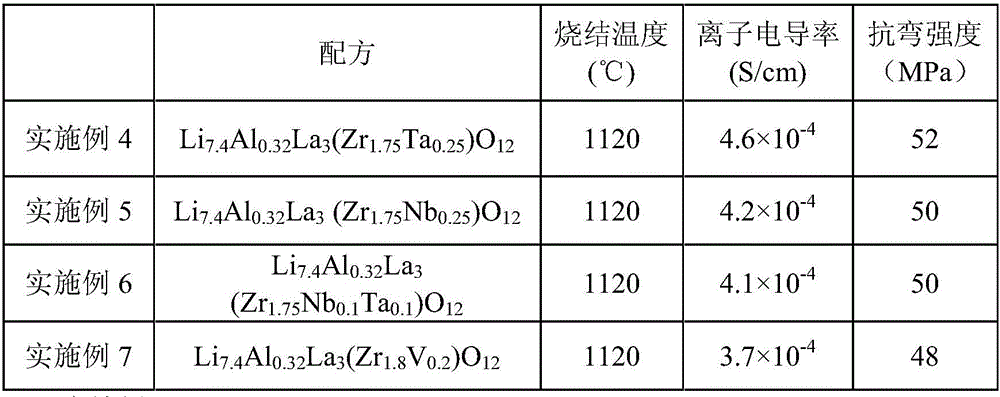
Embodiment 4-7
[0065] According to the general formula of chemical composition Li 6+x Al y La 3 (Zr n B 2-n )O 12 Ingredients, wherein B is at least one selected from Ti, Nb, Ta, Sb, V, and satisfies 0≤x≤2, 0<y≤1, 1≤n<2, using the same method as in Example 1-3 The preparation method prepares the solid electrolyte material.
[0066] See Table 2 for the chemical formulas and test results of the solid electrolyte materials in Examples 4-7.
[0067] The chemical formula and test result thereof of the solid electrolyte material of table 2 embodiment 4-7
[0068]
Embodiment 8-12
[0070] According to the general formula of chemical composition Li 6+x Al y (La z A 3-z )(Zr n G 2-n )O 12 Ingredients, wherein A is at least one selected from Ca, Sr, Y, and Ba, G is at least one selected from Ti, Nb, Ta, Sb, and V, and satisfies 0≤x≤2, 0
[0071] See Table 3 for the chemical formulas and test results of the solid electrolyte materials in Examples 8-12.
[0072] The chemical formula and test result thereof of the solid electrolyte material of table 3 embodiment 8-12
[0073]
[0074]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com