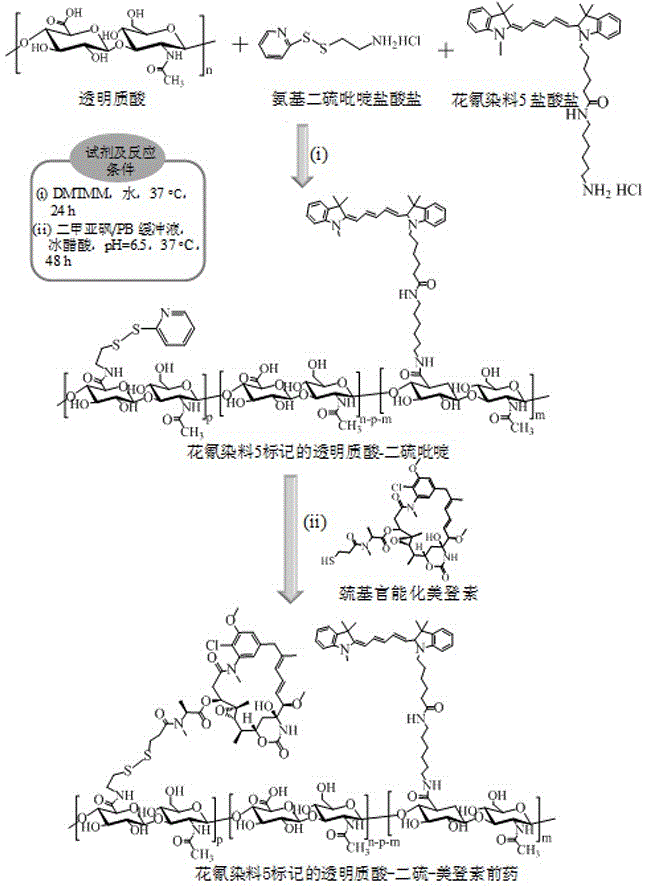
Hyaluronic acid derived maytansine prodrug, preparation method thereof and application of maytansine prodrug in preparation of tumor target treatment medicines
A technology of hyaluronic acid and maytansine, applied in anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve problems such as damage and interstitial lung disease, toxic and side effects, tissue damage, etc., and achieve preservation of anti-tumor activity, Good biological activity and the effect of maintaining medicinal properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
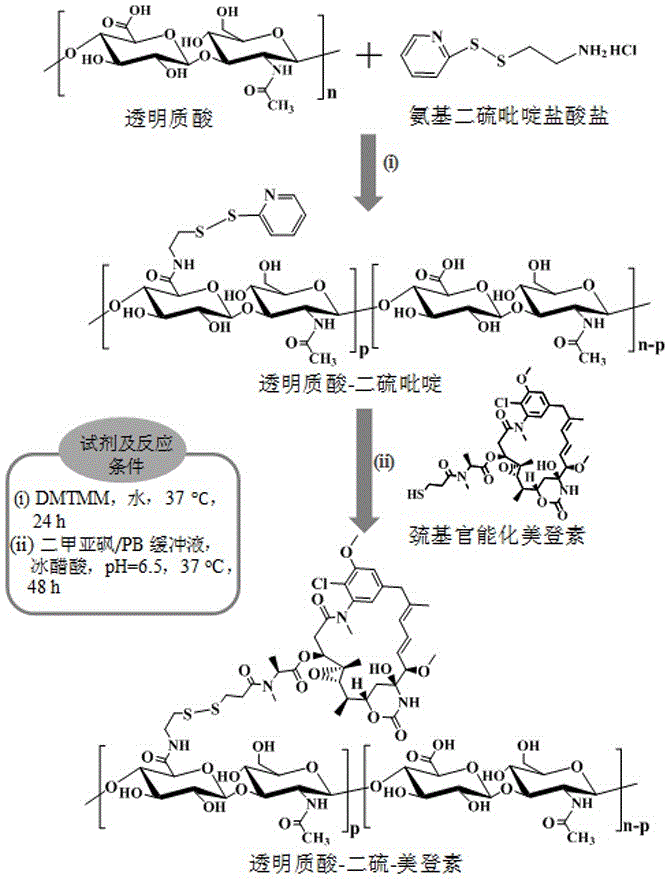
[0037] Example 1 Synthesis of hyaluronic acid derivatized maytansine prodrug (HA-SS-DM1) ( M nHA =35 kDa, DM1% =20 wt.%)
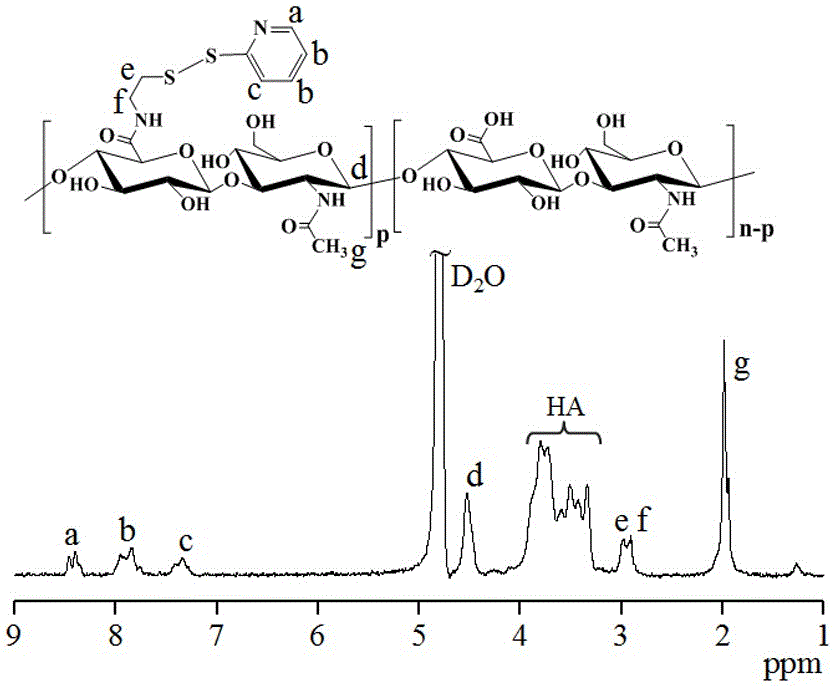
[0038] First, aminodithiopyridine hydrochloride (PDA·HCl) (34.7 mg, 0.156 mmol) was added to hyaluronic acid (HA) (400 mg, 1.04 mmol carboxyl) aqueous solution (20 mL) at room temperature and the whole solution was The pH was adjusted to 6.5, and 4-(4,6-dimethoxytriazin-2-yl)-4-methylmorpholine hydrochloride (DMTMM) (57.51 mg, 0.208mmol) was added thereto at 35 After stirring and reacting at ℃ for 24 hours, it was dialyzed and freeze-dried to obtain hyaluronic acid-dithiopyridine (HA-SS-Py). The product yield was 85%. NMR results showed that its structure was hyaluronic acid-dithiopyridine (HA-SS-Py), and the degree of substitution (DS) of the dithiopyridine functional group (-SS-Py) was 6%. NMR see image 3 , 1 H NMR (D 2 O): Hyaluronic acid (HA): δ (ppm) 1.86-2.01, 3.28-4.02, and 4.21-4.75; Dithiopyridine functional group (-SS-Py): δ (ppm) 2.90 (...
Embodiment 2
[0040] Example 2 Synthesis of hyaluronic acid derivatized maytansine prodrug (HA-SS-DM1) ( M nHA =35 kDa, DM1%=26 wt.%)
[0041] First, aminodithiopyridine hydrochloride (PDA•HCl) (53 mg, 0.24 mmol) was added to hyaluronic acid (HA) (400 mg, 1.04 mmol carboxyl) aqueous solution (20 mL) at room temperature and the whole solution The pH was adjusted to 6.5, and 4-(4,6-dimethoxytriazin-2-yl)-4-methylmorpholine hydrochloride (DMTMM) (86.3 mg, 0.316 mmol) was added thereto at 35 After stirring and reacting at ℃ for 24 hours, it was dialyzed and freeze-dried to obtain hyaluronic acid-dithiopyridine (HA-SS-Py). The product yield was 85%. NMR results showed that its structure was hyaluronic acid-dithiopyridine (HA-SS-Py), and the degree of substitution (DS) of the dithiopyridine functional group (-SS-Py) was 8.5%.
[0042] Under nitrogen protection, HA-SS-Py (100 mg, 40.6 micromole dithiopyridine functional group) dissolved in 8 mL of secondary water was sequentially added to a 1...
Embodiment 3
[0043] Example 3 Synthesis of hyaluronic acid derivatized maytansine prodrug (HA-SS-DM1) ( M nHA =35 kDa, DM1% =30 wt.%)
[0044] First, under nitrogen protection, add aminodithiopyridine hydrochloride (PDA•HCl) (70 mg, 0.312 mmol) to hyaluronic acid (HA) (400 mg, 1.04 mmol carboxyl) aqueous solution (20 mL) The pH of the solution was adjusted to 6.5, and then 4-(4,6-dimethoxytriazin-2-yl)-4-methylmorpholine hydrochloride (DMTMM) (115.01 mg, 0.416 mmol) was added thereto, After stirring and reacting at 35°C for 24 hours, it was dialyzed and freeze-dried to obtain hyaluronic acid-dithiopyridine (HA-SS-Py). The product yield was 85%. NMR results showed that its structure was hyaluronic acid-dithiopyridine (HA-SS-Py), and the degree of substitution (DS) of the dithiopyridine functional group (-SS-Py) was 11%.
[0045] Under nitrogen protection, add HA-SS-Py (100 mg, 54.2 micromole dithiopyridine functional group) dissolved in 8 mL of secondary water into a 100 mL three-necke...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com