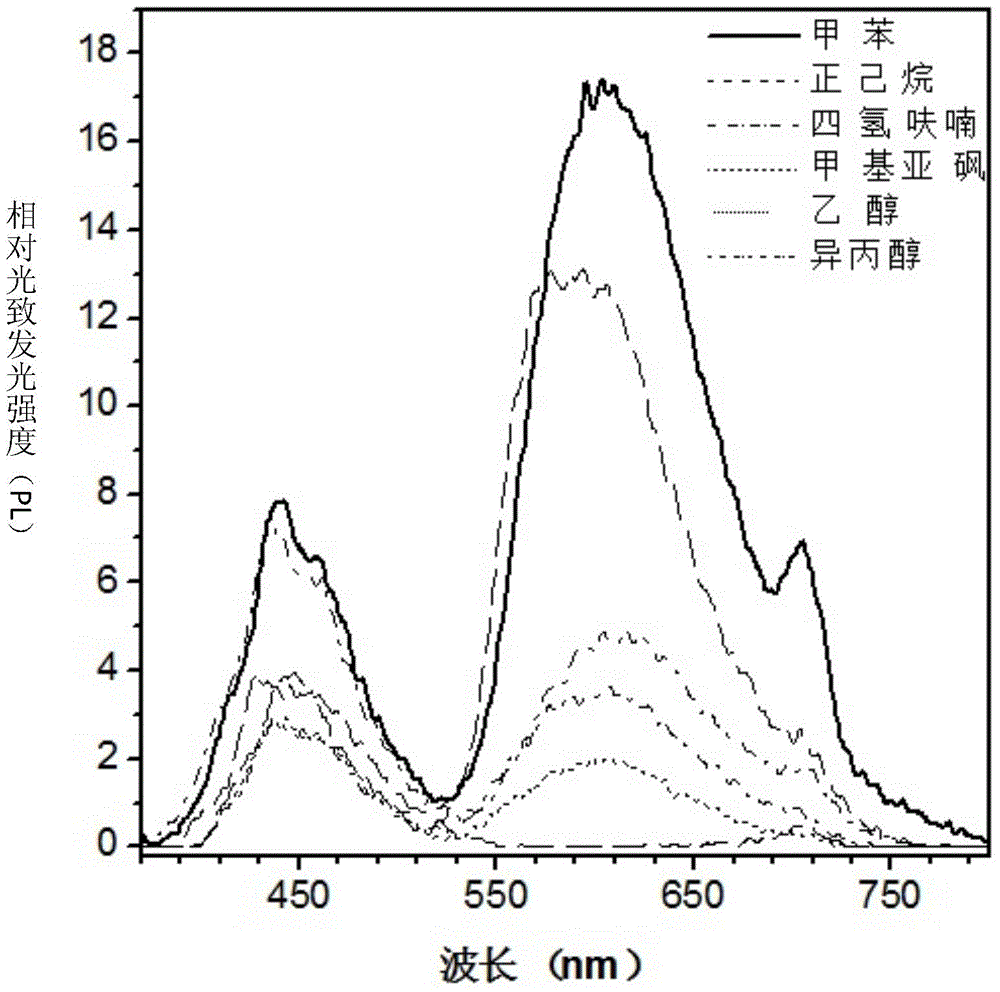
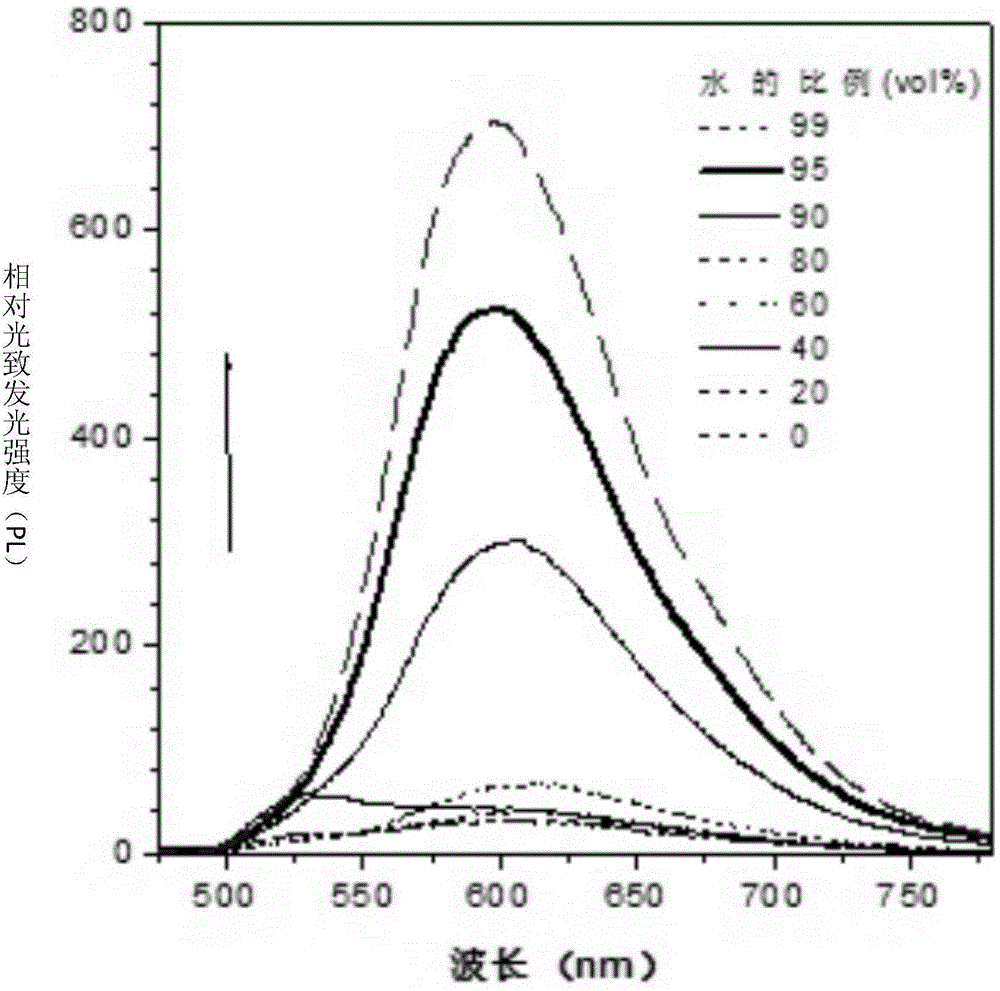
Fluorenyl salicylaldehyde buzane derivative and preparation method and application thereof
A fluorene-based salicylic and derivative technology, which is applied in biochemical equipment and methods, hydrazone preparation, and microbial determination/inspection, etc. Stiff displacement, the effect of preventing the self-absorption phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1: the synthesis (FAS) of fluorenyl salicylaldehyde hydrazine
[0066]
[0067] Reflux 10g of fluorenone and 20ml of hydrazine hydrate (85%) under 100ml for 4h. After cooling, a large number of needle-like solids precipitated. After filtering, recrystallize twice with ethanol to obtain light yellow needle-packed crystals with a purity of 99% and a yield of 95%. %; take 2g of hydrazine hydrate crystals and 2ml of salicylaldehyde in 20ml of ethanol to reflux for 4h, after cooling to obtain light yellow needle-packed crystals, after filtering, wash with 75% ethanol solution to obtain the FAS structure, the yield is 99%. MALDI-TOF(m / z):[M+]calcd.C 20 h 14 N 2 O, 298.34; found, 299.34. Anal Calc. for C 20 h 14 N 2 O: C, 80.52; H, 4.73; N, 9.39; O, 5.36. Found: C, 80.62; H, 4.71; N, 9.19; O, 5.36.
Embodiment 2
[0068] Embodiment 2: Synthesis of FAS derivatives (1)
[0069]
[0070] Get 500mg of hydrazine hydrate crystals and equimolar m-hydroxysalicylaldehyde (or m-bromosalicylaldehyde) derivatives to react in 10ml of ethanol at 60°C for 4h. After cooling, light yellow crystals are obtained. After filtration, use 75% ethanol solution After cleaning, the corresponding FAS-derived structure was obtained by chromatography of the product, and the yield was >90%.
[0071] m-FAS-OH:MALDI-TOF(m / z):[M+]calcd.C 20 h 14 N 2 o 2 ,314.34; found, 315.24.Anal Calc.for C 20 h 14 N 2 o 2 H, 4.49; N, 8.91; O, 10.18. Found: C, 75.32; H, 4.48; N, 8.35; O, 10.09.
[0072] p-FAS-Br:MALDI-TOF(m / z):[M+]calcd.C 20 h 13 BrN 2 O, 377.23; found, 299.34. Anal Calc. for C 20 h 13 BrN 2 O: C, 63.68; H, 3.47; Br, 21.18; N, 7.43; O, 4.24. Found: C, 63.58; H, 3.40;
Embodiment 3
[0073] Example 3: Synthesis of FAS derivatives (2)
[0074]
[0075] Take 500mg of hydrazine hydrate crystals and equimolar N,N-diethylaminosalicylaldehyde in 10ml of ethanol and react at 60°C for 4 hours. After cooling, a red oily substance is obtained. After filtering, wash with 75% ethanol solution to obtain the product layer The corresponding FAS derivative structure was obtained by analysis with a yield of 85%.
[0076] p-FAS-N22: MALDI-TOF(m / z):[M+]calcd.C 24 h 23 N 3 O: 369.18; found, 370.66; Anal Calc.for C 24 h 23 N 3 O: C, 78.02; H, 6.27; N, 11.37; O, 4.33. Found, C, 77.93; H, 6.10; N, 11.25; O, 4.45.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com