2, 3, 5, 6-tetrafluoro-7, 7', 8, 8'-tetracyanoquinodimethane and preparation method thereof
A technology of dimethyl p-benzoquinone, which is applied in the preparation of carboxylic acid nitriles, chemical instruments and methods, and the preparation of organic compounds, etc., can solve the problems of high toxicity, serious environmental pollution, and high risk of raw materials, and achieve easy operation , The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
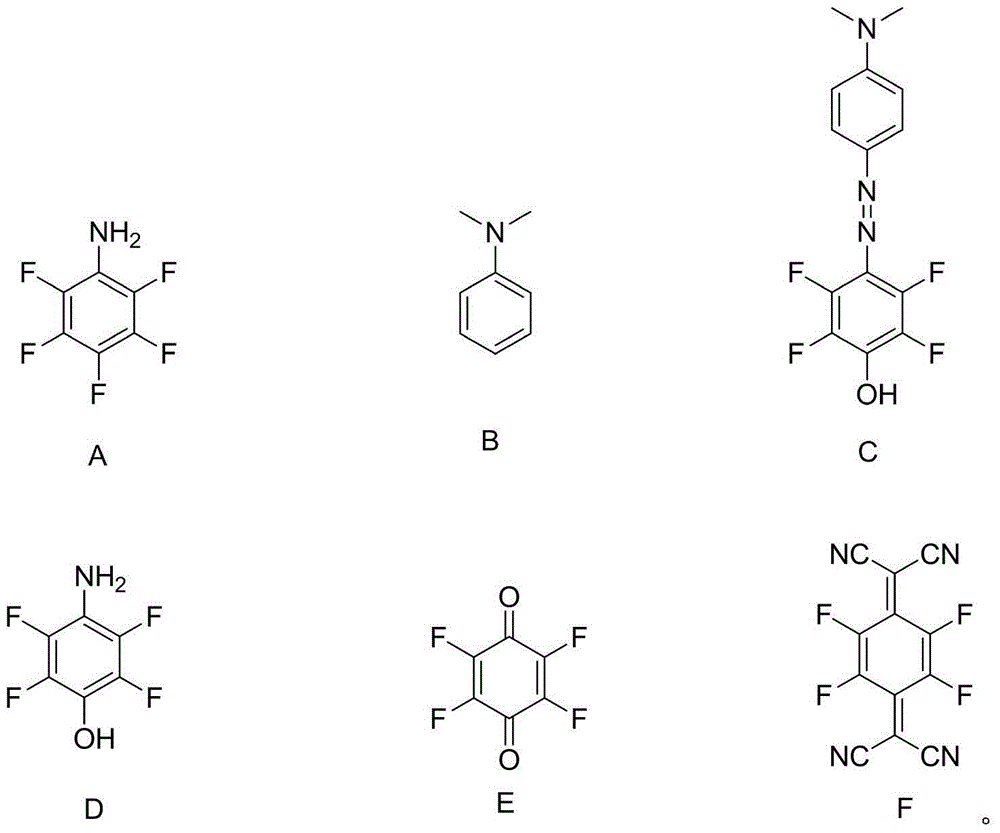
[0032] According to the preparation method of the present invention, the compound that undergoes diazotization reaction with compound A is preferably compound B (dimethylaminoaniline), and other anilines with similar structures, naphthylamine or other condensed ring aromatic amines can also be selected for use. After diazotization Compound D is obtained through a subsequent oxidation step. According to the preparation method of the invention, the process is simple and easy to operate, the yield is as high as over 80%, and the purity can reach over 99%.
[0033] According to one embodiment of the present invention, wherein in the diazotization step S1100, the diazotization reaction of compound A and compound B is: in the presence of nitrite and acid, -20°C to 50°C, more preferably 0 °C to -5 °C, more preferably 0 °C, compound A and compound B undergo a diazotization reaction. The acid is selected from at least one of the following: sulfuric acid, hydrochloric acid and phosphor...
Embodiment 1
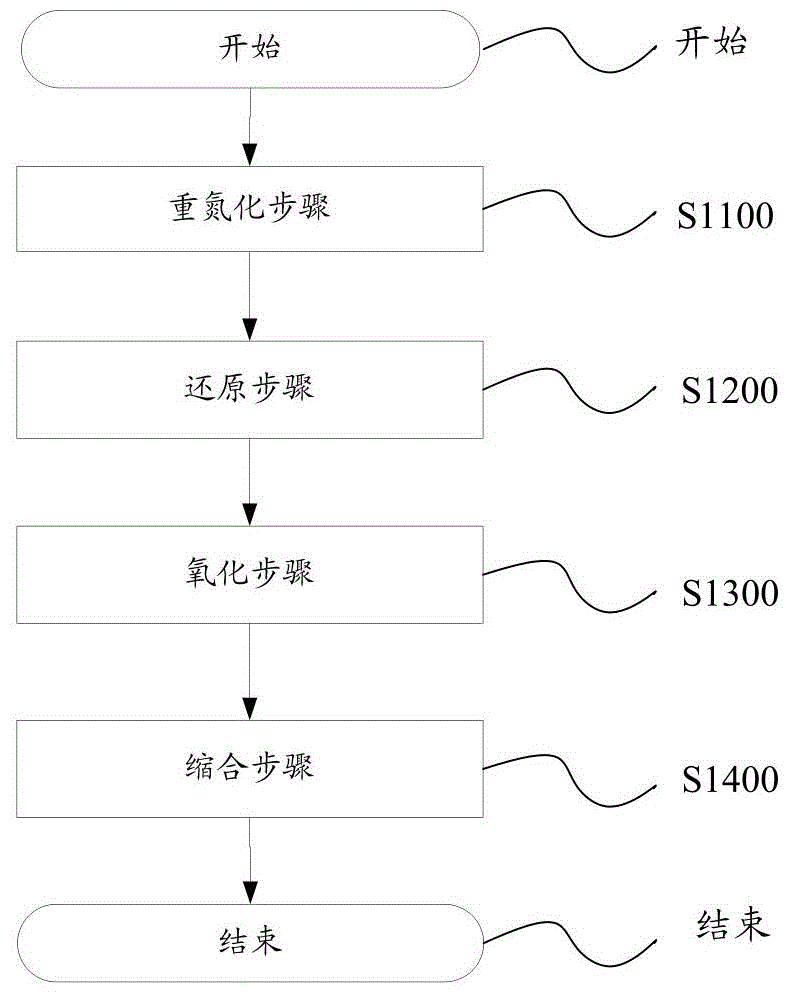
[0053] according to figure 1 The schematic flow chart of the preparation method shown, firstly carry out the diazotization step S1100: compound A undergoes a diazotization reaction with compound B to convert compound C; then enters the reduction step S1200: compound C is reduced and converted to compound D; then enters oxidation Step S1300: Compound D is oxidized and transformed into Compound E; finally enters the condensation step S1400: Compound E undergoes a condensation reaction with propanedicyanide and is transformed into Compound F (2,3,5,6-tetrafluoro-7,7',8 , 8'-tetracyanodimethyl-p-benzoquinone), so far the condensation step S1400 ends, and the preparation method is completed.
[0054] The specific operation of the diazotization step S1100 is as follows: 1eq compound A is dissolved in sulfuric acid aqueous solution containing 5eq sulfuric acid, the mass percentage of sulfuric acid aqueous solution is 70%; Sodium nitrate, stirred vigorously at this temperature for 1....
Embodiment 2
[0059] according to figure 1 In the flow chart of the preparation method shown, the diazotization step S1100 is first performed: compound A undergoes a diazotization reaction with compound B to convert compound C; then enters the reduction step S1200: compound C is reduced and converted to compound D; then enters the oxidation step S1300: Compound D is oxidized and transformed into Compound E; finally enters the condensation step S1400: Compound E undergoes a condensation reaction with propanedicyanide and is transformed into Compound F (2,3,5,6-tetrafluoro-7,7',8, 8'-tetracyanodimethyl-p-benzoquinone), so far the condensation step S1400 ends, and the preparation method is completed.
[0060] The specific operation of the diazotization step is as follows: 1eq compound A is dissolved in sulfuric acid aqueous solution containing 10eq sulfuric acid, the mass percentage of sulfuric acid aqueous solution is 70%; then cool down to -5°C, add 2eq sodium nitrite in batches while stirri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com