Application of chemical components of Herba Ecliptae to phytoestrogens
A technology of Eclipta chinensis extract and estrogen, which can be applied to medical preparations containing active ingredients, plant raw materials, organic active ingredients, etc., can solve problems such as large side effects, increased incidence of uterine cancer and breast cancer, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of active ingredient in embodiment 1 Eclipta
[0034] Take 20kg Eclipta chinensis (purchased from Anguo Shengshan Pharmaceutical Co., Ltd., batch number: mhl0001, place of origin: Hebei), and extract with 100L of 70% ethanol under heat reflux, each extraction time is 2 hours, a total of 3 extractions. Distilled under reduced pressure at 45°C, concentrated to no alcohol, and the estimated extract mass is about 1kg.
[0035] Dissolve the crude extract in 10L of water, and extract 3 times with an equal amount of petroleum ether and ethyl acetate to obtain 255g of petroleum ether extract (named EPA-P), and 150g of ethyl acetate extract (named EPA-P). EPA-E), aqueous extract 600g (designated as EPA-W).
[0036] The water layer part (EPA-W) 600g, is separated by adsorption of macroporous resin D101, adopts pure water, 30% ethanol, 70% ethanol, pure ethanol for elution, and 30% ethanol eluate is EPA-W-D3.
[0037] The EPA-W-D3 components are 25g in total, mi...
Embodiment 2
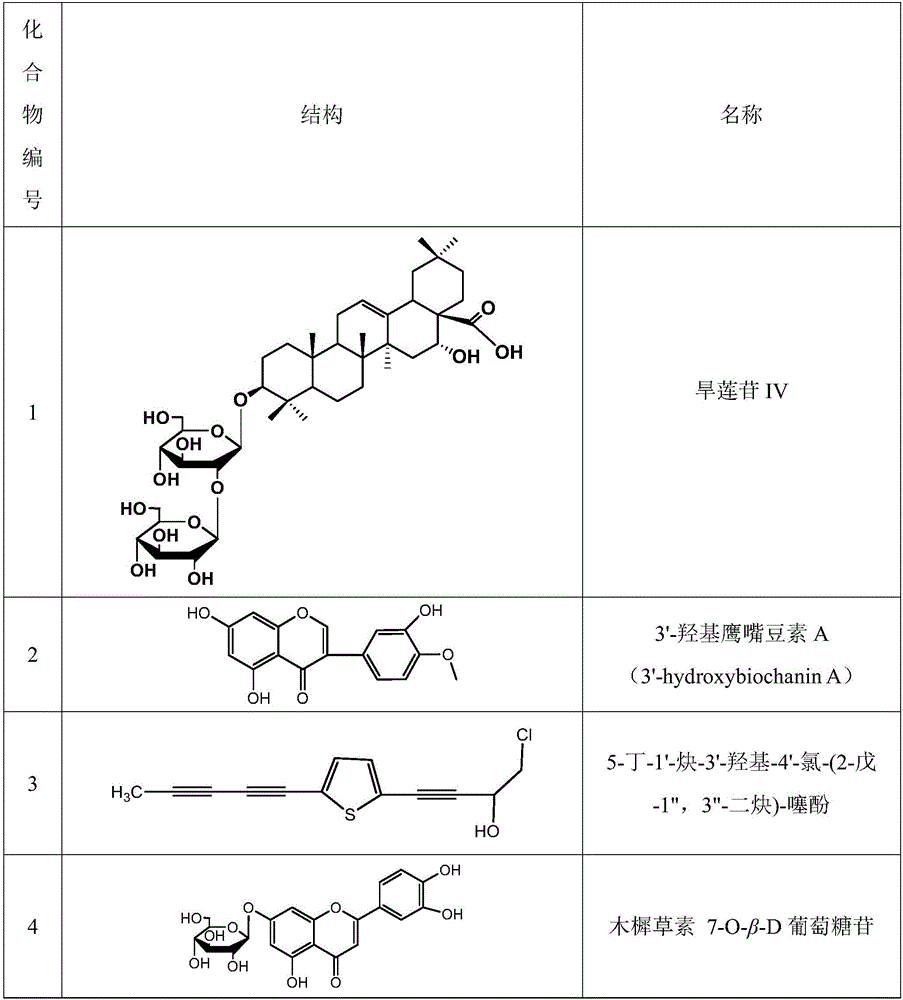
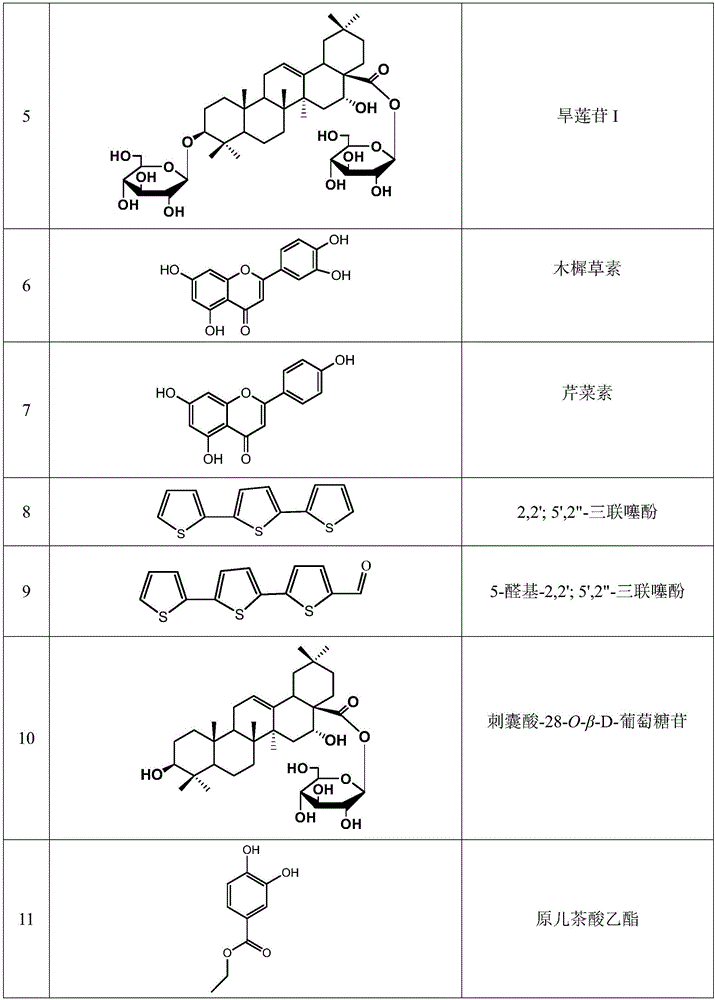
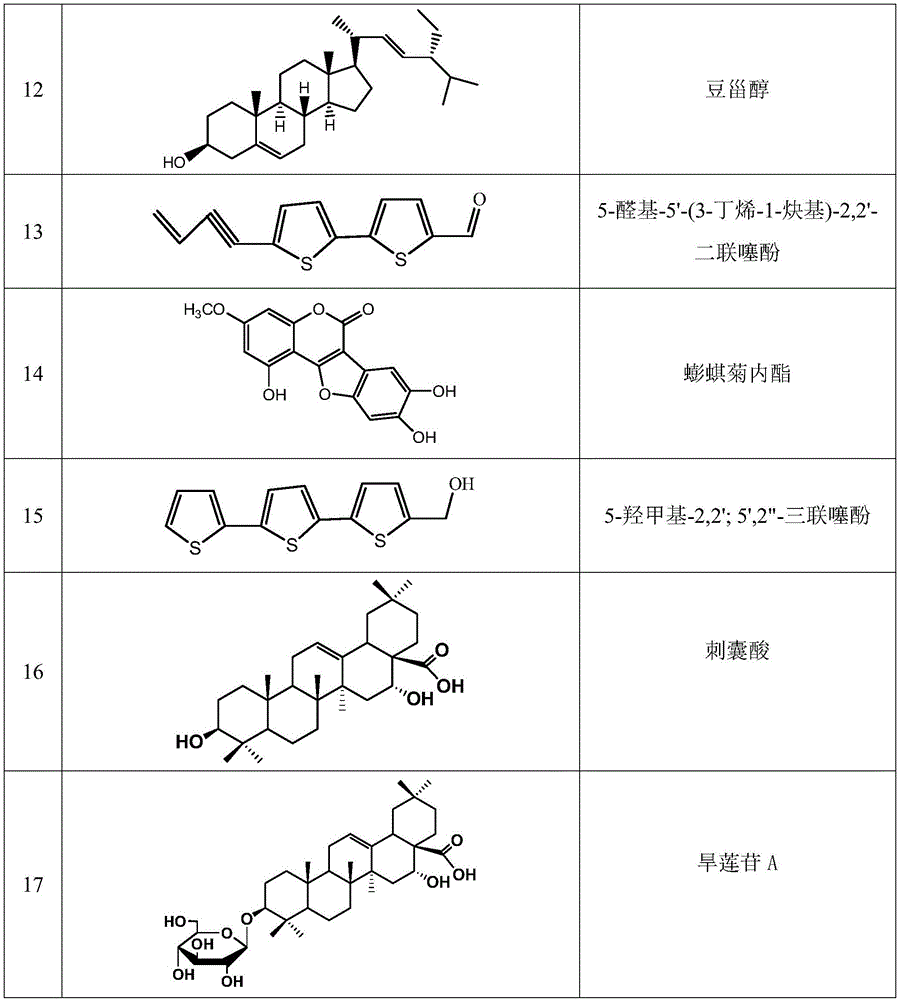
[0042] Embodiment 2: Determination of the structure of compound 1-17
[0043] pass 1 H. 13 C-NMR determined the structure of compound 1-17; the determined structure of compound 1-17 is shown in Table 1.
[0044] The model of the NMR instrument is: BRUKER AVANCE Ⅲ 500 superconducting nuclear magnetic resonance spectrometer (TMS is the internal standard, Swiss Bruker company);
[0045] specific:
[0046] Compound 1 was characterized as follows:
[0047] 1 H-NMR (500MHz, pyridine-d 5 ) There are 2 hydrogens in the low field region of the spectrum: δ5.65 (1H, br s, H-12) and 5.25 (1H, br s, H-16). It can be observed in the middle and low field areas: δ3.31 (1H, m) is the H-3 hydrogen signal; δ4.92 (1H, d, J = 7.0Hz, H-1') and δ5.37 (1H, d , J=7.5Hz, H-1") suggest that there are two glucose groups. In the high field area, seven characteristic methyl peak signals can also be observed: δ1.85 (3H, s, H-27), 1.28 (3H,s,H-23),1.19(3H,s,H-30),1.10(3H,s,H-24),1.07(3H,s,H-29),1.0...
Embodiment 3
[0084] The mensuration of compound content in the extract of Eclipta chinensis in embodiment 3
[0085] (a) preparation of reference substance and need testing solution
[0086] Weigh an appropriate amount of each reference substance, weigh them accurately, put them in a 10mL volumetric flask, add methanol-water solution with a volume fraction of 50% to the mark, and obtain the reference substance series solution ①, luteolin 7-O-β-D glucoside (abbreviated as A, 18.15mg), luteolin (abbreviated as B, 4.64mg), eclidein IV (referred to as C, 11.85mg), apigenin (abbreviated as D, 1.56mg), ecliptaside A (referred to as E, 21.50 mg), echinocysic acid-28-O-β-D-glucoside (referred to as F, 2.60mg), echinocysic acid (referred to as G, 8.45mg). Take 1mL each of the reference substance series solutions ① and place them in 10mL volumetric flasks, add methanol-water solution with a volume fraction of 50% to the mark to obtain the reference substance series solutions ②. Take each referen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com