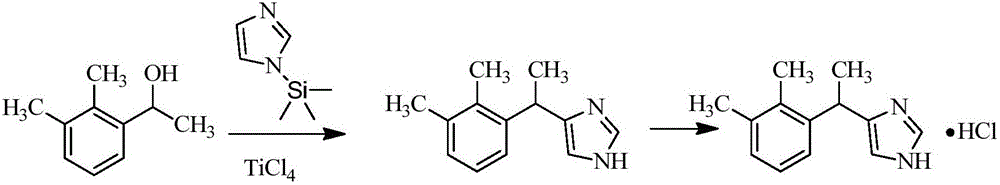
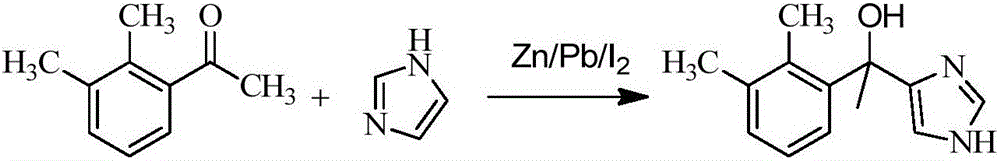
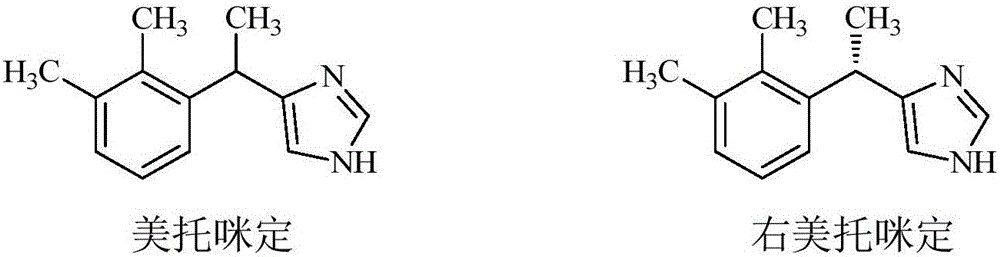Preparation method of dexmedetomidine and intermediate product thereof
A technology of dexmedetomidine and asana, which is applied in the field of preparation of dexmedetomidine and intermediates thereof, and can solve the problems of long synthesis process route, cumbersome processing technology, inconsistency with green chemistry and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Preparation of 1-(1-bromoethyl)2,3-dimethylbenzene
[0058]
[0059] In a 5L three-necked flask, add 1L of tetrahydrofuran and 60g (2.5mol) of magnesium strips, heat to reflux, and add dropwise 750mL of a tetrahydrofuran solution of 463g (2.5mol) of 2,3-dimethylbromobenzene. After reflux for 1 hour, cool to room temperature, add dropwise acetaldehyde 180ml (3.2mol) / tetrahydrofuran 500mL solution, continue to reflux for 1 hour, remove tetrahydrofuran under reduced pressure, and slowly add ammonium chloride aqueous solution (125g ammonium chloride to 400ml water) and Ethyl acetate 1.5L. Stand to separate layers, dry the organic phase with anhydrous sodium sulfate, filter, distill off the solvent, and then distill under reduced pressure to collect fractions at 115-118°C / 300Pa. Dissolve 330 g of the distilled product in 1.5 L of dichloromethane, cool down to 0°C, add phosphorus tribromide (1188 g, 4.4 mol) in 1 L of dichloromethane solution dropwise, and stir at room te...
Embodiment 3
[0063] Example 3: Preparation of 4-[(2,3-dimethylphenyl)-ethyl]-1-(triphenylmethyl)imidazole
[0064]
[0065] Preparation of starting material 1: Weigh compound 1 (52.3g, 0.12mol), add 300ml tetrahydrofuran / water (2:1), mark A solution for future use;
[0066] Preparation of starting material 2: Weigh compound 2 (21.2g, 0.1mol), add 300ml tetrahydrofuran / water (2:1), then add 52.9g potassium phosphate, mark solution B for later use;
[0067] Prepare catalyst 0.5g PdCl 2 [dtbpf] tetrahydrofuran solution, add 10ml tetrahydrofuran mark C solution mark C solution;
[0068] Use a pump to inject solution A and solution B into the micromixer at a flow rate of 25ul / min, and at the same time add solution C to it at the same rate, and after mixing, enter the microtube reactor with a diameter of 0.5mm and a tube length of 1.5m for reaction. The oil temperature in the microtube reactor was 65°C, and it was detected by an HPLC detector. The solution containing the compound of formula I...
Embodiment 4
[0069] Example 4: Preparation of 4-[(2,3-dimethylphenyl)-ethyl]-1-(triphenylmethyl)imidazole
[0070]
[0071] Under argon atmosphere, add compound 1 (26.15g, 0.06mol), compound 2 (10.6g, 0.05mol) and 150mL tetrahydrofuran / water (2:1) to a 5L reaction flask, and then add 26.45g potassium phosphate , 0.25g PdCl 2 [dtbpf], after 2-3 times of argon replacement, microwave heating to 65°C, after 10 minutes, HPLC detected that the reaction was complete, filtered, concentrated under reduced pressure and evaporated to remove the solvent, and purified by column chromatography to obtain 19.74g, yield 95.1% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com