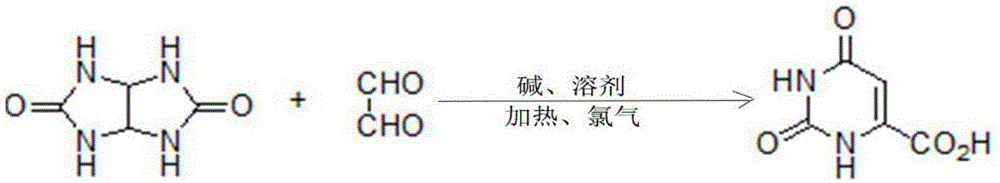
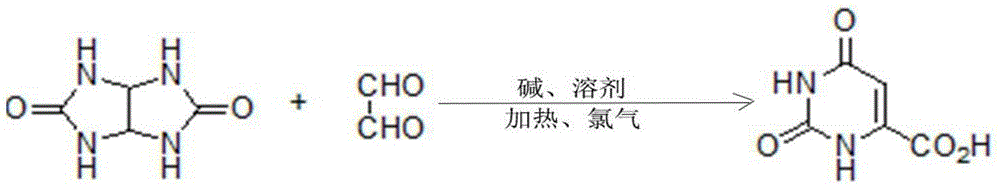
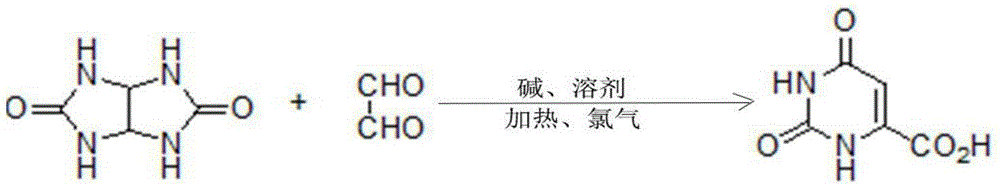
Method for synthesizing orotic acid
A synthetic method and technology of orotic acid, applied in the direction of organic chemistry, can solve the problems of complex reaction conditions, expensive bromine, high cost, etc., and achieve the effect of simple reaction conditions, high product purity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: A kind of synthetic method of orotic acid, the substrate glycoluril and glyoxal are condensed in alkaline solvent and then pass into chlorine gas one-pot reaction to obtain orotic acid, after the reaction finishes, cool to normal temperature, Use acid to adjust the pH value of the solution to 0, filter, and the obtained solid is crude orotic acid; suspend the crude product in water, add concentrated ammonia water dropwise at a temperature of 60°C until the solid is completely dissolved, and then adjust to weak acidity with sulfuric acid, Add activated carbon and filter while it is hot, adjust the filtrate to strong acidity, cool and crystallize, filter and dry to obtain refined orotic acid. Wherein, the molar mass ratio of the glycoluril and glyoxal is 1:1; the temperature of the reaction is 0°C, the alkali is sodium hydroxide, the molar mass ratio of the alkali to the substrate is 1:1, and the chlorine gas and the alkali The molar mass ratio of the solven...
Embodiment 2
[0027] Embodiment 2: A kind of synthetic method of orotic acid, the substrate glycoluril and glyoxal are condensed in alkaline solvent and then passed into chlorine gas one-pot reaction to obtain orotic acid, after the reaction finishes, cool to normal temperature, Use acid to adjust the pH value of the solution to 6, filter, and the obtained solid is crude orotic acid; suspend the crude product in water, add concentrated ammonia water dropwise at a temperature of 100°C until the solid is completely dissolved, and then adjust it to weak acidity with hydrochloric acid, Add activated carbon and filter while it is hot, adjust the filtrate to strong acidity, cool and crystallize, filter and dry to obtain refined orotic acid. Wherein, the molar mass ratio of the glycoluril and glyoxal is 1:2; the reaction temperature is 150°C, the alkali is barium hydroxide, the molar mass ratio of the alkali to the substrate is 1:10, and the chlorine gas and the alkali The molar mass ratio of the ...
Embodiment 3
[0028] Embodiment 3: a kind of synthetic method of orotic acid, the substrate glycoluril and glyoxal are condensed in alkaline solvent and then passed into chlorine gas one-pot reaction to obtain orotic acid, after the reaction finishes, cool to normal temperature, Use acid to adjust the pH value of the solution to 1, filter, and the obtained solid is crude orotic acid; suspend the crude product in water, add concentrated ammonia water dropwise at a temperature of 72°C until the solid is completely dissolved, and then adjust it to weak acidity with sulfuric acid, Add activated carbon and filter while it is hot, adjust the filtrate to strong acidity, cool and crystallize, filter and dry to obtain refined orotic acid. Wherein, the molar mass ratio of the glycoluril and glyoxal is 1:1.4; the temperature of the reaction is 15°C, the alkali is lithium hydroxide, the molar mass ratio of the alkali to the substrate is 1:2, and the chlorine gas and the alkali The molar mass ratio of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com