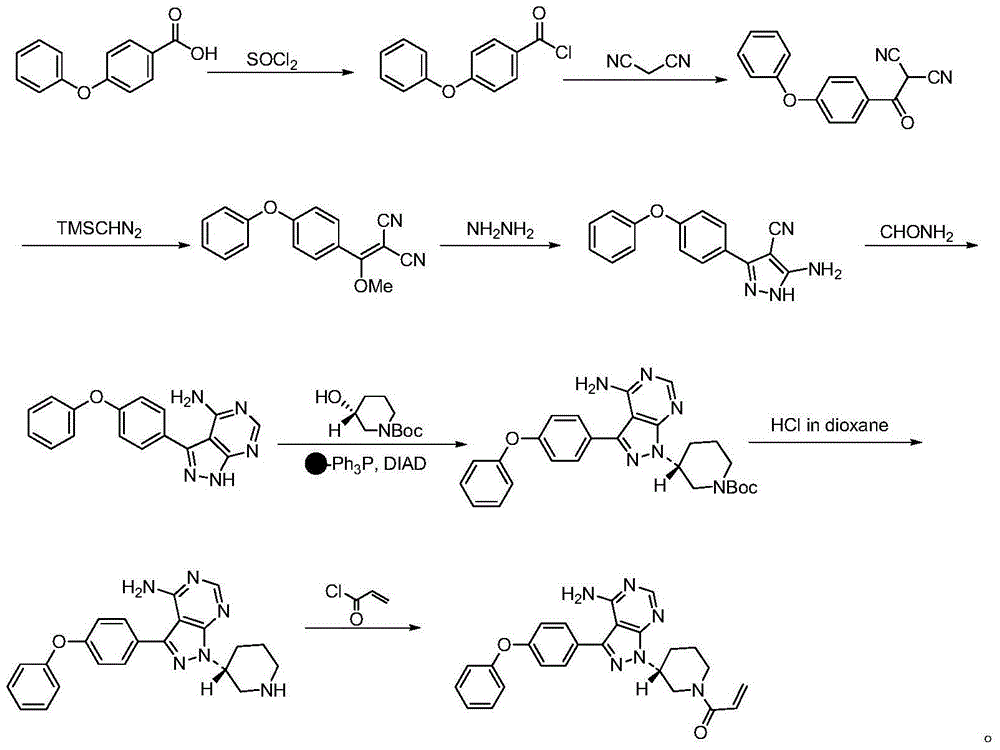
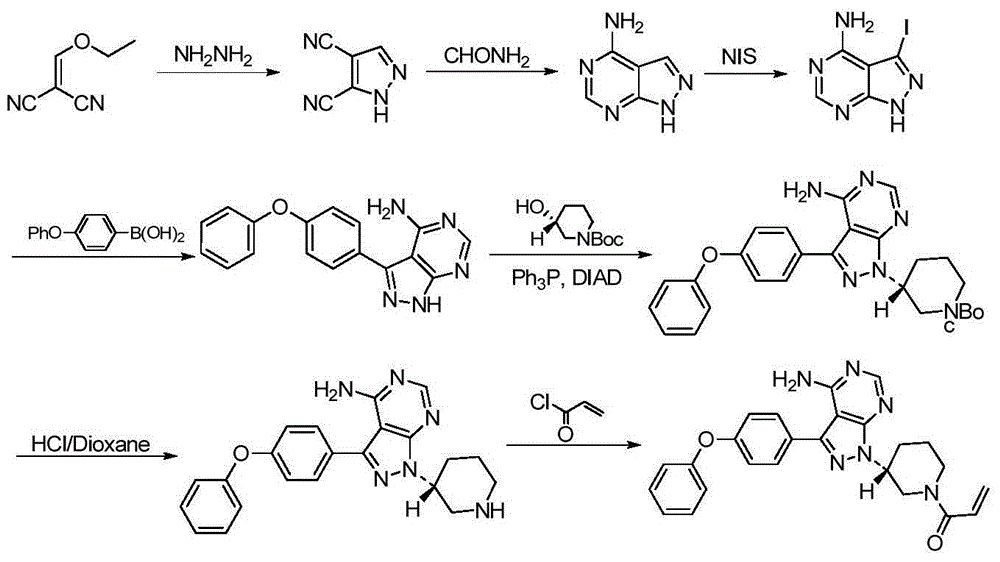
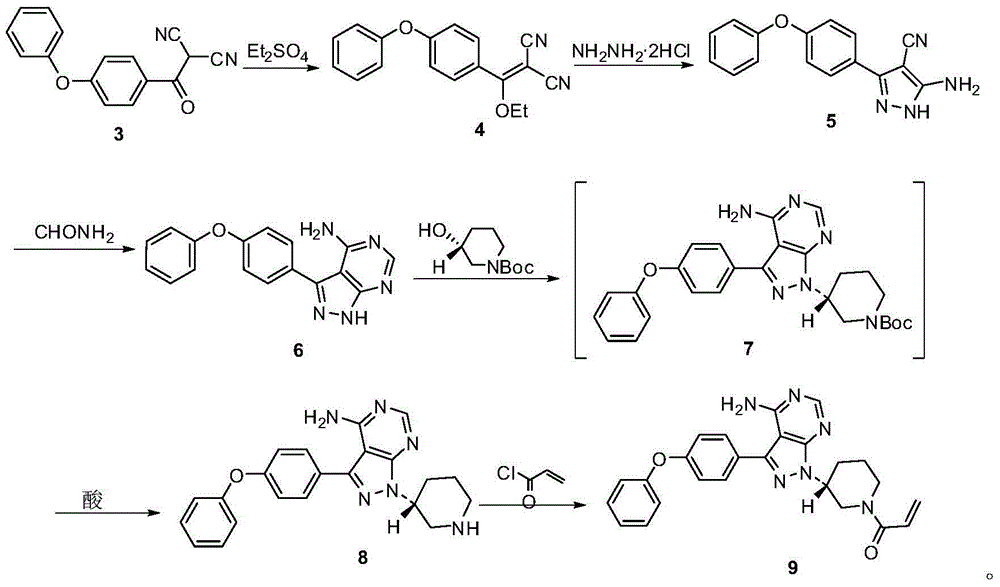
Ibrutinib preparation method, ibrutinib intermediate, and ibrutinib intermediate preparation method
A technology of ibrutinib and intermediates, applied in the field of small molecule drugs, can solve problems such as difficult methylation reactions, hidden dangers of heavy metal residues, and reduced market competitiveness, to achieve low prices, increase reaction yields, and reduce production costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Synthetic compound (2)
[0054]
[0055]Measure toluene (4 Vol, volume multiple relative to the mass of compound 1) into the reaction kettle, and weigh 1 eq of compound (1) into the above reaction kettle. Thionyl chloride (1.5eq) was added, and the reaction was heated at 110°C until the conversion of the raw material was complete. The solvent was removed by rotary evaporation to obtain a crude product, which was directly used in the next reaction.
Embodiment 2
[0057] Synthetic compound (3)
[0058]
[0059] Prepare a solution of malononitrile (1.1eq) in Me-THF (1Vol), dropwise add it to a solution of NaH (2eq) in Me-THF (2Vol), and continue stirring for 30min after the addition of malononitrile is complete. 1eq of compound (2) was dissolved in Me-THF (2Vol) to form a solution, which was added dropwise to the above reaction solution. After the dropwise addition was completed, stirring was continued until the reaction was completed. Add 1N hydrochloric acid to the reaction solution to pH = 2-3, add water (4 Vol) for separation. The aqueous phase was extracted twice with EA (3 Vol), and the organic phases were combined and spin-dried to obtain a crude product. The crude product was purified by recrystallization with a yield of 95%.
[0060] The NMR data of the product are as follows:
[0061] 1 H NMR (400MHz, DMSO) δ7.67–7.59(m,2H),7.48–7.37(m,2H),7.19(t,J=7.4Hz,1H),7.09–7.02(m,2H),6.99– 6.91 (m,2H).
Embodiment 3
[0063] Synthetic compound (4)
[0064]
[0065] Weigh compound 3 (1eq), add 1,4-Dioxane (5vol), and stir to dissolve. Weigh NaHCO 3 (1.5eq), was added to the reaction kettle. The system was heated to 70°C, and Et was added dropwise 2 SO 4 (2eq). HPLC monitors that the reaction is completed, and the temperature is cooled. EA (4vol) was extracted and washed with saturated brine (4vol). Dried and spin-dried to obtain the crude product, which was directly used in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com