A kind of preparation method of chitosan grafted cetirizine nano-micelle
A cetirizine and nanomicelle technology, which is applied in the directions of non-active ingredient medical preparations, active ingredients-containing medical preparations, pharmaceutical formulas, etc. problems, to achieve the effect of good research and development application prospects, simple preparation technology, and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
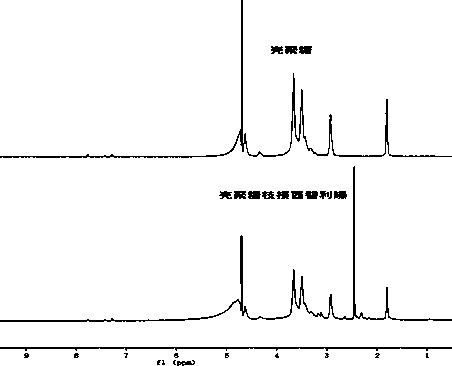
[0015] 3. Preparation of chitosan grafted cetirizine
[0016] Weigh chitosan and dissolve in dilute acid solution, wherein the concentration of dilute acid solution is controlled at 0.5-2% (v / v), the concentration of chitosan solution is 0.2-1%, and chitosan solution is added drop by drop into the carboxyl-activated cetirizine solution and stirred evenly, wherein the molar ratio of chitosan to cetirizine is 1:0.5-2, and the reaction is continued for 12-48h.
[0017] 4. Purification of the product
[0018] The reaction product is dialyzed in deionized water, and the light yellow solid obtained by drying is the chitosan cetirizine graft.
[0019] 5. Preparation of chitosan-grafted cetirizine nanomicelles
[0020] Dissolve the chitosan cetirizine graft in dilute acid solution, where the concentration of dilute acid is controlled at 1-2% (v / v), and perform ultrasonication (750W, 20-40%, 6-24min) , that is, chitosan grafted cetirizine nano-micelle.
[0021] The dilute acid solu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com