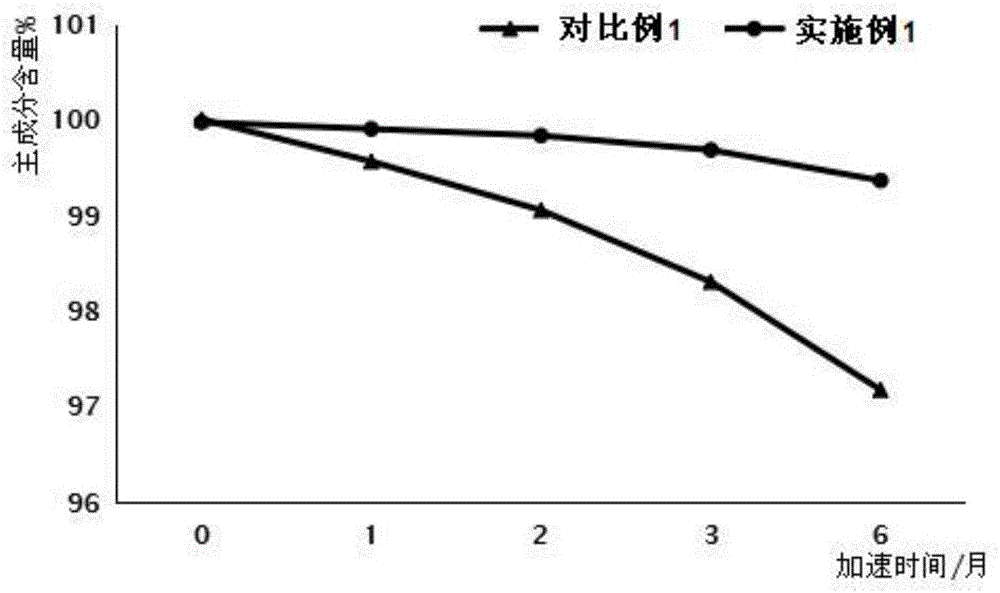
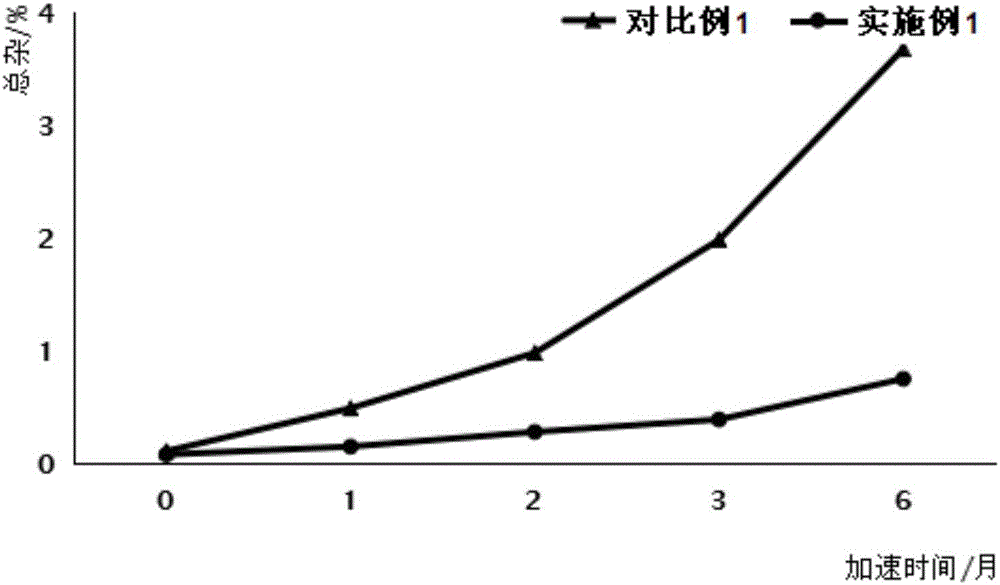
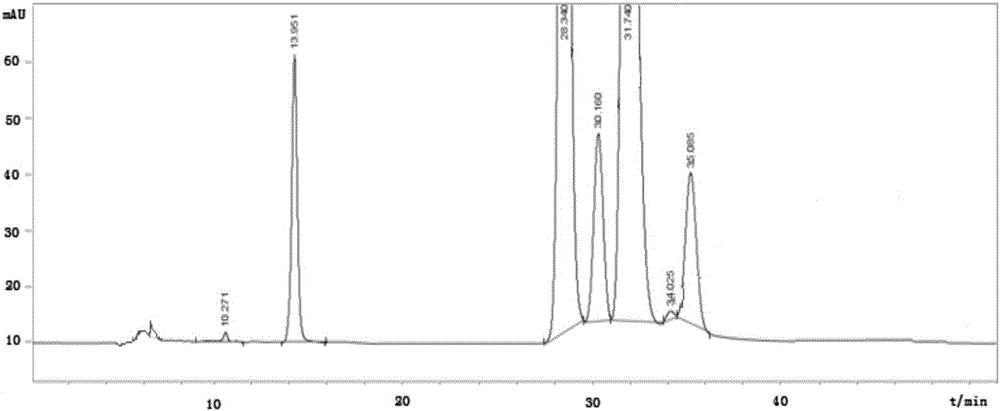
Silybin meglumine preparation
A technology of thisbin meglumine and silybin, which is applied in the direction of pill delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of shortening the validity period of products, reducing the content and dissolution rate Fast and other problems, to achieve the effects of increasing drug efficacy, accelerating disintegration, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0045] In the present invention, the binder is used to bind the drug powder together to facilitate the molding of the preparation. It can adopt various common binders used in the preparation of silybin meglumine in the prior art, or it can be The specific adhesive used in the present invention is not particularly limited here. As a preferred embodiment of the present invention, in terms of mass percentage, the adhesive contains the following components:
[0046] Starch 50-75%,
[0047] Gum Arabic 10-20%,
[0048] Carboxymethylcellulose 5-8%,
[0049] Polyvinyl alcohol 1-3%,
[0050] Polypropylene glycol 3-10%,
[0051] Alginic acid 5-12%,
[0052] The sum of the above components is 100%.
[0053] As a preferred embodiment of the present invention, the mass percentage of the binder is composed of 65% starch, 12% gum arabic, 7% carboxymethyl cellulose, 2% polyvinyl alcohol, 6% polypropylene glycol, alginic acid 8%.
[0054] In the present invention, the disintegrating ag...
Embodiment 1
[0067] In this embodiment, the composition of the silibinin meglumine preparation is: the proportion of silibinin meglumine is 10%, the diluent is 80%, the binder is 4.5%, and the disintegrant is 3.25%, the lubricant is 0.25%, and the dispersant is 2%. Among them, the diluent is a mixture of dextrin and lactose in a mass ratio of 2:1; the binder is composed of 65% starch, 12% gum arabic, 7% carboxymethyl cellulose, 2% polyvinyl alcohol, polypropylene glycol 6 %, alginic acid 8%; the disintegrating agent is sodium carboxymethyl starch; the lubricant is magnesium stearate; the dispersing agent is a mixture of stearamide and methyl pentanol at a mass ratio of 1:1.
[0068] In this example, the preparation method of the silibinin meglumine preparation is as follows: first mix the silibinin meglumine, diluent, and dispersant uniformly according to the aforementioned ratio, and then co-grind until the average particle size of the powder is Diameter is lower than 100 μ m, obtain ult...
Embodiment 2
[0079] In this embodiment, the composition of the silibinin meglumine preparation is: 15% silybin meglumine, 70% diluent, 5% binder, 3.75% disintegrant, 6% dispersant , lubricant 0.25%. Wherein, the diluent is a mixture of dextrin and lactose in a mass ratio of 2:1; the binder consists of the following components: 60% starch, 15% gum arabic, 5% carboxymethyl cellulose, polyvinyl alcohol 3%, polypropylene glycol 7%, alginic acid 10%; the disintegrant is sodium starch glycolate; the dispersant is a 1:1 mixture of stearamide and methyl amyl alcohol by mass; the lubricant for magnesium stearate.
[0080] In this example, the preparation method of the silybin meglumine preparation is as follows: first mix the silibinin meglumine, diluent, and dispersant uniformly according to the aforementioned ratio, and then carry out co-grinding until the average particle size of the powder is Diameter is less than 100 μ m, obtain superfine powder, then add binder in proportion to mix and gran...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com