Ionic liquid with amino acids and preparation method and application of ionic liquid
A technology of ionic liquids and amino acids, applied in chemical instruments and methods, catalytic reactions, organic chemistry, etc., can solve the problems that conventional ionic liquids cannot meet the needs, achieve high yield, ensure catalytic effect, and reduce costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
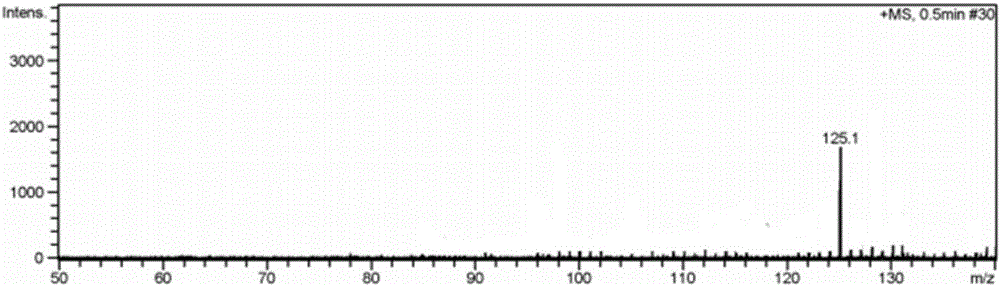
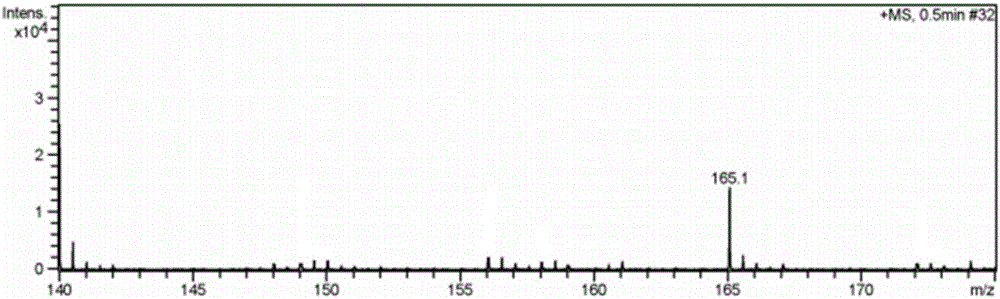
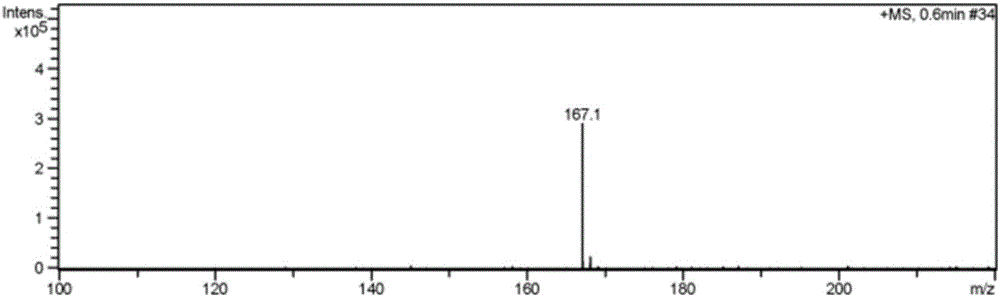
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of cationic amino acid ionic liquid without amino groups
[0029] (1) Preparation of 1-butyl-3-methylimidazolium aspartate ionic liquid ([bmim][ASP])
[0030] N-methylimidazole and n-butane bromide with a molar ratio of 1:1.2 were mixed and dissolved in 100 mL of toluene solution, and the reaction was refluxed in an oil bath at 70 °C for 8 h. After the reaction, put it into the refrigerator to freeze, and crystallize for 24h. Filtration under reduced pressure and vacuum drying at 80°C for 24h; after obtaining 1-butyl-3-methylimidazolium bromide ionic liquid, the bromine was exchanged for -OH on the resin through 717 type anion exchange resin, and then the exchanged The ionic liquid reacted with aspartic acid in deionized water at a molar ratio of 1:1.1, stirred for 24 h, filtered to remove excess amino acid, the filtrate was concentrated by rotary evaporator under reduced pressure distillation, suction filtered, and vacuum dried at 60 °C After 24...
Embodiment 2
[0041] Example 2 Preparation of cationic amino acid ionic liquid with amino group
[0042](1) 1-Aminopropyl-3-butylimidazolium aspartic acid ionic liquid ([NH 2 pbim][ASP]) preparation
[0043] Under nitrogen protection, 3-bromopropylamine hydrobromide and n-butylimidazole were mixed in a molar ratio of 1:1.1, dissolved in a certain amount of anhydrous ethanol solution, condensed and refluxed at 90 °C for 24 hours, and distilled under reduced pressure to remove After ethanol solvent, vacuum-drying, adding an appropriate amount of KOH aqueous solution to make the pH of the solution 8-9, drying, adding anhydrous ethanol solvent to the solid, stirring to dissolve, filtering, and vacuum-drying the filtrate to obtain 1-aminopropyl-3 After -butylimidazolium bromide ionic liquid, the bromine is exchanged for -OH on the resin through 717 type anion exchange resin, and then the exchanged ionic liquid is reacted with aspartic acid in deionized water at a molar ratio of 1:1.1 , stirred...
Embodiment 3
[0054] Example 3 Catalytic cycloaddition reaction of epoxy compounds by cationic amino acid ionic liquid without amino group
[0055] (1) The effect of temperature on the reaction yield
[0056] In a 50ml autoclave, add catalyst [bmim][ASP] and epichlorohydrin, mix, and feed 0.5MPCO 2 , at the temperature shown in Table 1, the reaction was carried out for 12 hours. The amount of catalyst added is 0.3% of the moles of epichlorohydrin. After the reaction, the yield of cyclic carbonate was calculated, and the results are shown in Table 1.
[0057] Table 1
[0058] temperature °C
[0059] (2) CO 2 Effect of pressure on reaction yield
[0060] The method is the same as (1), the temperature is 130 °C, and the pressure is changed as shown in Table 2. The results are shown in Table 2.
[0061] Table 2
[0062] pressure MP
[0063] (3) Influence of catalyst dosage on reaction yield
[0064] The method is the same as (1), the temperature is 130 °C, the amount...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com