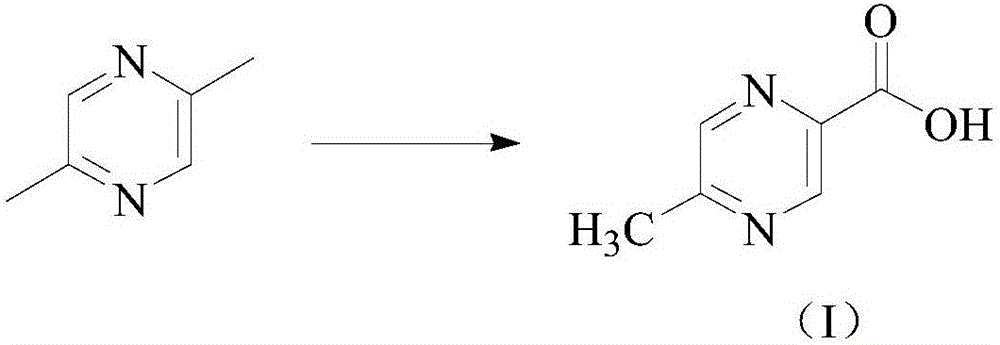
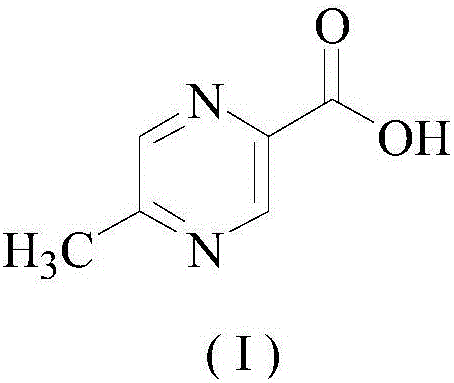
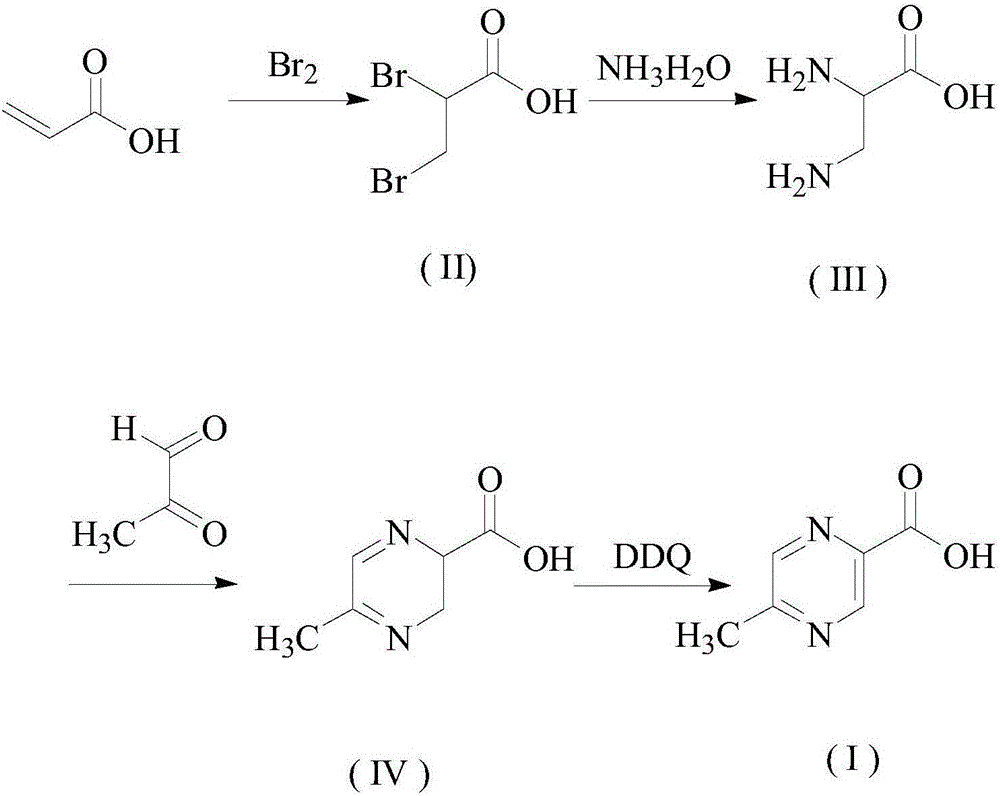
Preparation method of pyrazine carboxylic acid
A technology of pyrazine carboxylic acid and acrylic acid, applied in the direction of organic chemistry and the like, can solve the problems of waste of resources, unfavorable environmental protection production, difficult separation and the like, and achieves the effects of mild conditions and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 20g of acrylic acid and 200mL of dichloromethane to a 500mL three-necked flask in sequence, start stirring, control the temperature at 20-30°C and slowly add 51.1g of bromine dropwise, and the dropwise addition is completed in 15 minutes. Heat to 30-50°C and stir under temperature control for 2 hours, stop heating, add 200mL saturated sodium chloride solution, separate the liquids, and lower the temperature to 0°C. Stir and crystallize for 4h, filter to obtain intermediate (II);
[0031] S2: Add the intermediate (II) obtained in step S1 into 200 mL of absolute ethanol, slowly add 50 mL of ammonia water dropwise at room temperature, react at room temperature for 6 hours, and evaporate the solvent to obtain intermediate (III);
[0032] S3: Add the intermediate (III) obtained in step S2 and 20g of aceglyoxal into a 500mL reaction flask, add 200mL of absolute ethanol, stir to make it completely dissolve; heat up to reflux, reflux for 4h, and evaporate the solvent under ...
Embodiment 2
[0035] Add 20g of acrylic acid and 200mL of dichloromethane to a 500mL three-necked flask in sequence, start stirring, control the temperature at 20-30°C and slowly add 44.4g of bromine dropwise, and the dropwise addition is completed in 10 minutes. Heat to 30°C and stir under temperature control for 2.5h, stop heating, add 200mL saturated sodium chloride solution, separate the liquids, and lower the temperature to 0°C. Stir and crystallize for 3h, filter to obtain intermediate (II);
[0036] S2: Add the intermediate (II) obtained in step S1 into 200 mL of absolute ethanol, slowly add 30 mL of ammonia water dropwise at room temperature, react at room temperature for 5 hours, and evaporate the solvent to obtain intermediate (III);
[0037] S3: Add the intermediate (III) obtained in step S2 and 15g of aceglyoxal into a 500mL reaction flask, add 200mL of absolute ethanol, stir to make it completely dissolve; heat up to reflux, reflux for 3h, and evaporate the solvent under reduce...
Embodiment 3
[0040] Add 20g of acrylic acid and 200mL of dichloromethane to a 500mL three-necked flask in sequence, start stirring, control the temperature at 20-30°C and slowly add 66.7g of bromine dropwise, and the dropwise addition is completed in 20 minutes. Heat to 50°C and stir under temperature control for 1.5h, stop heating, add 200mL of saturated sodium chloride solution, separate the liquids, and lower the temperature to 0°C. Stir and crystallize for 5h, filter to obtain intermediate (II);
[0041] S2: Add the intermediate (II) obtained in step S1 into 200 mL of absolute ethanol, slowly add 100 mL of ammonia water dropwise at room temperature, react at room temperature for 7 hours, and evaporate the solvent to obtain intermediate (III);
[0042] S3: Add the intermediate (III) obtained in step S2 and 30g of aceglyoxal into a 500mL reaction flask, add 200mL of absolute ethanol, stir to make it completely dissolve; heat up to reflux, reflux for 5h, and evaporate the solvent under re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com