A kind of organic dye sensitizer containing bodipy type conjugated unit and preparation method thereof
A technology of organic dyes and sensitizers, applied in the field of dye-sensitivity solar cell materials, can solve the problems of non-planar three-dimensional structure weakening the absorption of light, and achieve easier control of reaction conditions, low production cost, and common and readily available raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Synthesis of Dye 1:
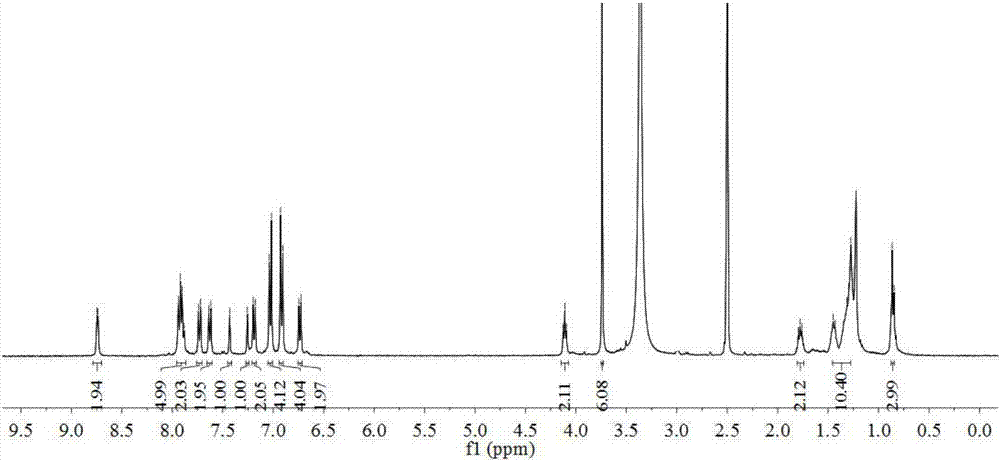
[0088] Intermediate 12 (0.16 g, 0.2 mmol), cyanoacetic acid (0.04 g, 0.4 mmol), 20 mL of chloroform and 20 mL of acetonitrile were sequentially added into a 100 mL three-neck flask. Install a reflux condenser, vacuumize, replace the argon protection, quickly add 2 drops of piperidine, and heat up to 80°C for reflux reaction for 24h. Stop the reaction, cool to room temperature, add 100 mL of dichloromethane to dilute the reaction solution, wash the organic phase with saturated brine three times (100 mL×3), and dry over anhydrous magnesium sulfate overnight. The filtrate was collected by filtration, the solvent was removed under reduced pressure, and the crude product was purified by silica gel (300-400 mesh) column chromatography [eluent, V (dichloromethane): V (methanol) = 10:1] to obtain a dark green solid powder Dye1 (0.14 g), 80% yield. 1 H NMR (400MHz, DMSO-d 6 )δ: 8.74(d, J=4.4Hz, 2H), 7.94–7.88(m, 5H), 7.73(d, J=8.5Hz, 2H), 7.63(d, J=8.6Hz...
Embodiment 2
[0090] Synthesis of Dye 2:
[0091] Intermediate 13 (0.16g, 0.2mmol) and cyanoacetic acid (0.04g, 0.4mmol) were sequentially added into a 100mL three-neck flask. A green solid powder Dye2 (0.13 g) was obtained by a method similar to the synthesis of Dye1 with a yield of 78%. 1 H NMR (400MHz, DMSO-d 6 )δ: 8.69(d,J=17.6Hz,1H),7.94–7.86(m,3H),7.78(d,J=8.2Hz,1H),7.74–7.69(m,1H),7.65(dd,J =10.0,4.4Hz,2H),7.56–7.38(m,7H),7.35–7.27(m,4H),7.18(dd,J=11.7,3.0Hz,3H),7.09–7.02(m,2H), 6.27(d, J=4.0Hz, 1H), 6.22(d, J=9.0Hz, 1H), 4.12(t, J=8.1Hz, 2H), 3.77(d, J=7.3Hz, 3H), 1.81– 1.76(m,2H),1.46–1.28(m,10H),0.88(s,3H). 13 C NMR (100MHz, DMSO-d 6 )δ:163.74,161.36,155.86,149.96,147.29,143.78,142.28,140.98,140.15,137.41,135.99,135.06,134.22,133.33,131.61,130.71,130.11,129.94,128.27,127.95,127.64,126.02,125.83,124.14 ,115.36,110.97,102.87,68.39,55.94,31.72,29.50,29.18,29.05,26.01,22.57,14.43.MALDI-TOF-MS,m / z:calcd.forC 54 h 47 BF 2 N 4 o 4 :864.370,found:864.212[M] + .
Embodiment 3
[0093] Synthesis of Dye3:
[0094] Intermediate 14 (0.16g, 0.2mmol) and cyanoacetic acid (0.04g, 0.4mmol) were sequentially added into a 100mL three-neck flask. A green solid powder Dye3 (0.13 g) was obtained by a method similar to the synthesis of Dye1 with a yield of 75%. 1 H NMR (400MHz, DMSO-d 6 )δ:8.82(s,1H),8.28–8.22(m,2H),8.02–7.93(m,4H),7.78(d,J=8.6Hz,1H),7.72–7.69(m,1H),7.56 (d, J=8.8Hz, 1H), 7.49(s, 1H), 7.42(t, J=7.8Hz, 4H), 7.31(d, J=7.8Hz, 4H), 7.27–7.21(m, 4H) ,7.06–7.03(m,1H),4.14(t,J=6.3Hz,2H),3.97–3.79(m,3H),1.85–1.71(m,2H),1.52–1.28(m,10H),0.91 (t,J=6.9Hz,3H). 13 C NMR (100MHz, DMSO-d 6 )δ:163.47,162.03,159.03,146.70,143.85,141.84,141.62,139.42,139.14,135.38,133.47,132.46,132.06,130.76,130.12,129.93,128.87,126.25,125.86,124.06,122.80,122.51,121.45,120.92 ,120.43,118.67,115.83,115.45,110.04,106.82,68.38,55.90,31.74,29.48,29.17,29.10,26.02,22.58,14.45. 52 h 45 BF 2 N 4 o 4 :838.350,found:838.991[M] + .
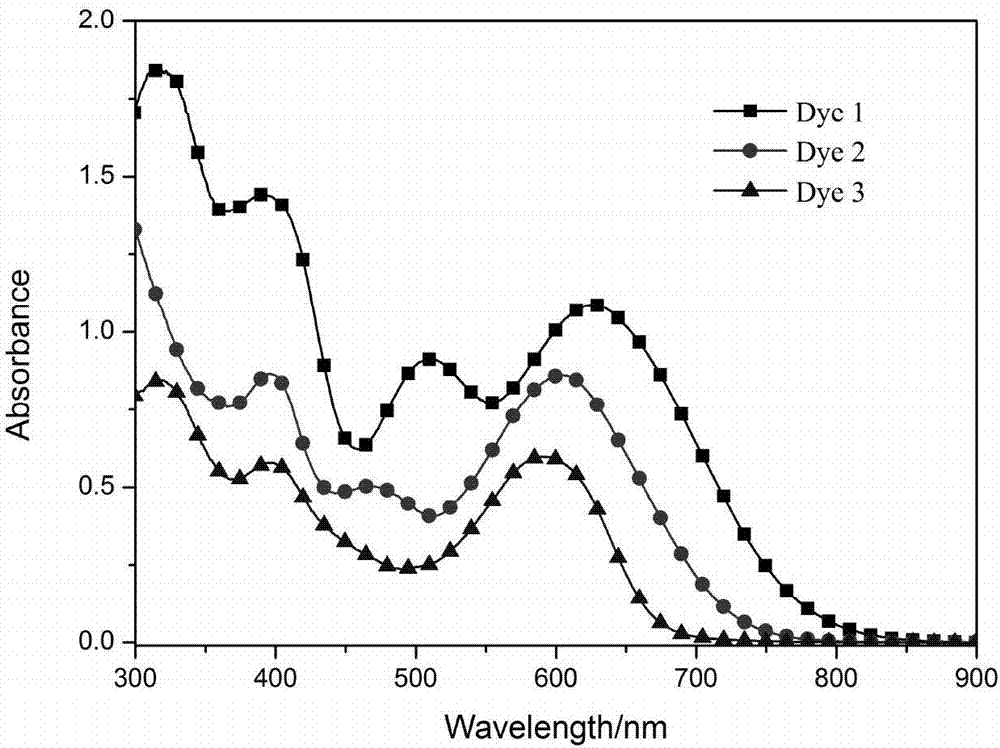
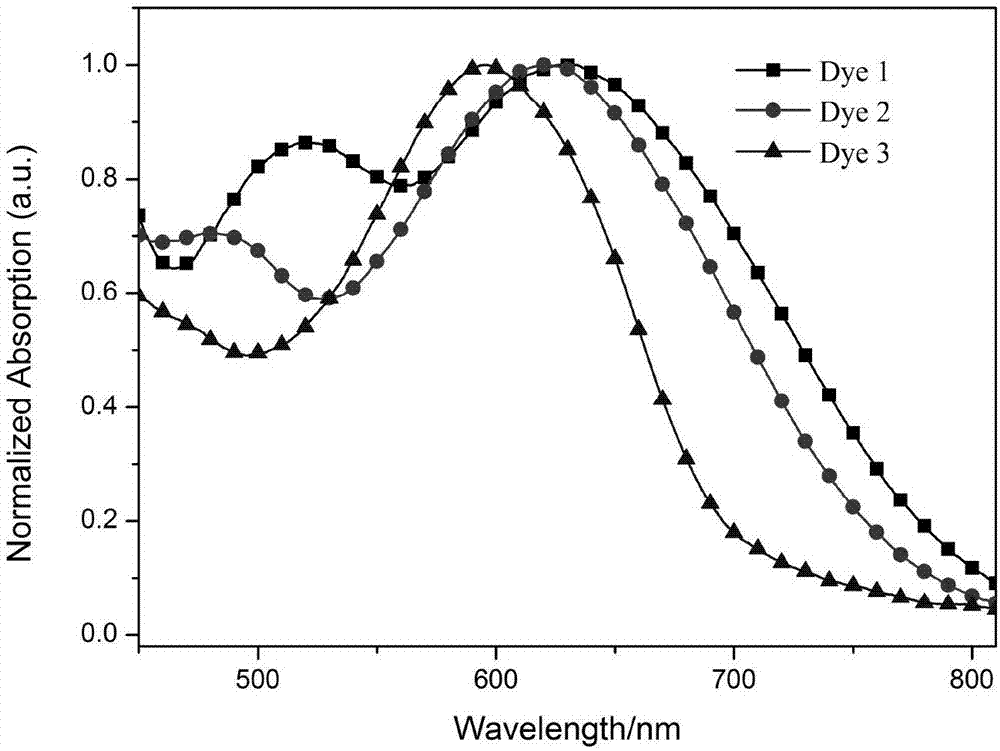
[0095] The relevant data of the UV absorptio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com