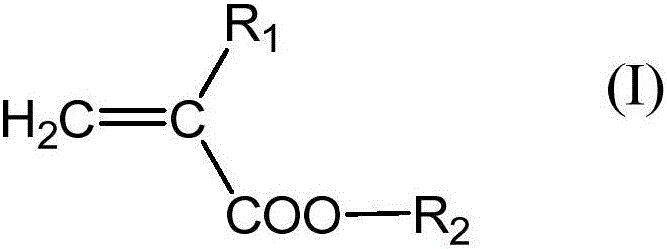
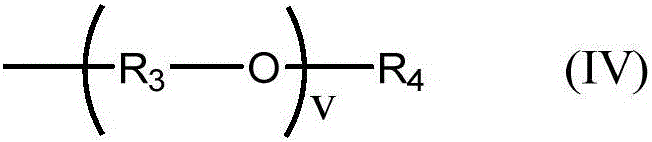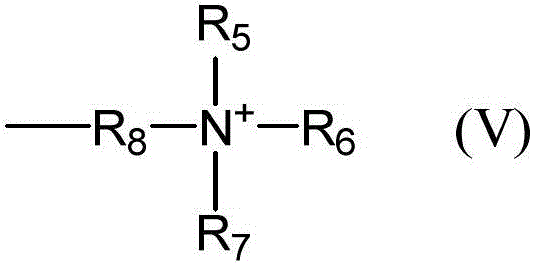Binder for lithium cell
A technology for binders and lithium batteries, applied in the field of binders and compositions for electrode production, can solve the problems of reduced charge and discharge capacity, large volume change, electrode damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0306] Synthesis of Synthesis Example 1 Crosslinked Copolymer P-01
[0307] 10 g of propylene glycol monomethyl ether acetate (manufactured by Wako Pure Chemical Industries, Ltd.) was added to a 200 ml round-bottomed flask equipped with a stirring device, a condenser, a thermometer, and a nitrogen inlet tube, and heated to an internal temperature of 90° C. under a nitrogen stream. Next, 9.2 g of acrylic acid (0.13 mol, manufactured by Wako Pure Chemical Industries, Ltd.), 0.78 g of 2-hydroxyethyl acrylate (6.7 mmol, manufactured by Tokyo Chemical Industry Co., Ltd.), 0.03 g of diethylene glycol diallyl ether ( A solution obtained by mixing 0.18 mmol (manufactured by Wako Pure Chemical Industries, Ltd.) and 13 g of propylene glycol monomethyl ether acetate was dropped into a round-bottomed flask over 2 hours. Thereafter, the resulting solution was reacted at 90° C. for 2 hours. After the reaction, it was cooled to room temperature, and 30 g of propylene glycol monomethyl eth...
Synthetic example 2
[0308] Synthesis of Synthesis Example 2 Crosslinked Copolymer P-02
[0309] Except that 0.68 g of dimethylacrylamide (6.8 mmol, manufactured by Wako Pure Chemical Industries, Ltd.) was used instead of 0.78 g of 2-hydroxyethyl acrylate, polymerization was carried out in the same manner as in Synthesis Example 1 to obtain cross linked copolymers. This was made crosslinked copolymer P-02 (acrylic acid / dimethylacrylamide=95 / 5).
Synthetic example 3
[0310] Synthesis of Synthesis Example 3 Crosslinked Copolymer P-03
[0311] Instead of 9.2 g of acrylic acid and 0.78 g of 2-hydroxyethyl acrylate, 7.5 g of acrylic acid (0.10 mol) and 2.5 g of methoxypolyethylene glycol methacrylate (5.4 mmol, manufactured by Shinnakamura Chemical Co., Ltd.) were used. Otherwise, the polymerization reaction was carried out in the same manner as in Synthesis Example 1 to obtain a crosslinked copolymer. This was used as crosslinked copolymer P-03 (acrylic acid / methoxypolyethylene glycol methacrylate=95 / 5).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com