Preparation method of nitrogen-doped carbon aerogel applied to lithium-ion battery
A lithium-ion battery, carbon aerogel technology, applied in electrical components, battery electrodes, circuits, etc., can solve the problems of wide explosion limit of ammonia gas, dangerous operation process, etc., and achieve a simple and effective preparation method, excellent conductivity, high The effect of energy density and power density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Weigh resorcinol and melamine in a certain proportion, add 1.2g / L NaOH solution and stir, then add 37wt% formaldehyde solution, stir at 60°C until clear. Wherein, the molar ratio of resorcinol to melamine is 9:1; the molar ratio of NaOH to (resorcinol+melamine) is 1:100, and formaldehyde: (resorcinol+melamine)=2:1.
[0029] (2) The clear solution obtained in (1) was transferred to a sealed container, gelled at 50° C. for 1 day, and aged at 80° C. for 5 days to obtain a wet gel.
[0030] (3) The wet gel obtained in (2) was soaked in acetone solution for solvent replacement, and the acetone was renewed every 8 hours for a total of 6 times.
[0031] (4) drying the wet gel obtained in (3) at 50° C. to obtain an aerogel.
[0032] (5) Put the aerogel obtained in (4) into a tube furnace, and carbonize at 700° C. for 3 h to obtain nitrogen-doped carbon aerogel. The nitrogen content of the material is 2.82 at%.
Embodiment 2
[0034] (1) Weigh resorcinol and melamine in a certain proportion, add 1.2g / L NaOH solution and stir, then add 37wt% formaldehyde solution, stir at 60°C until clear. Among them, the molar ratio of resorcinol to melamine is 7:3; the molar ratio of NaOH to (resorcinol+melamine) is 1:100, and formaldehyde: (resorcinol+melamine)=2:1.
[0035] (2) Transfer the clear solution obtained in (1) to a sealed container, gel at 50°C for 1 day, and age at 80°C for 5 days to obtain a wet gel.
[0036] (3) The wet gel obtained in (2) was soaked in acetone solution for solvent replacement, and the acetone was renewed every 8 hours for a total of 6 times.
[0037] (4) drying the wet gel obtained in (3) at 50° C. to obtain an aerogel.
[0038] (5) Put the aerogel obtained in (4) into a tube furnace, and carbonize at 700° C. for 3 h to obtain nitrogen-doped carbon aerogel. The nitrogen content of the material is 3.82 at%.
Embodiment 3
[0040] (1) Weigh resorcinol and melamine in a certain proportion, add 1.2g / L NaOH solution and stir, then add 37wt% formaldehyde solution, stir at 60°C until clear. Wherein, the molar ratio of resorcinol to melamine is 5:5; the molar ratio of NaOH to (resorcinol+melamine) is 1:100, and formaldehyde: (resorcinol+melamine)=2:1.
[0041] (2) The clear solution obtained in (1) was transferred to a sealed container, gelled at 50° C. for 1 day, and aged at 80° C. for 5 days to obtain a wet gel.
[0042] (3) The wet gel obtained in (2) was soaked in acetone solution for solvent replacement, and the acetone was renewed every 8 hours for a total of 6 times.
[0043] (4) drying the wet gel obtained in (3) at 50° C. to obtain an aerogel.
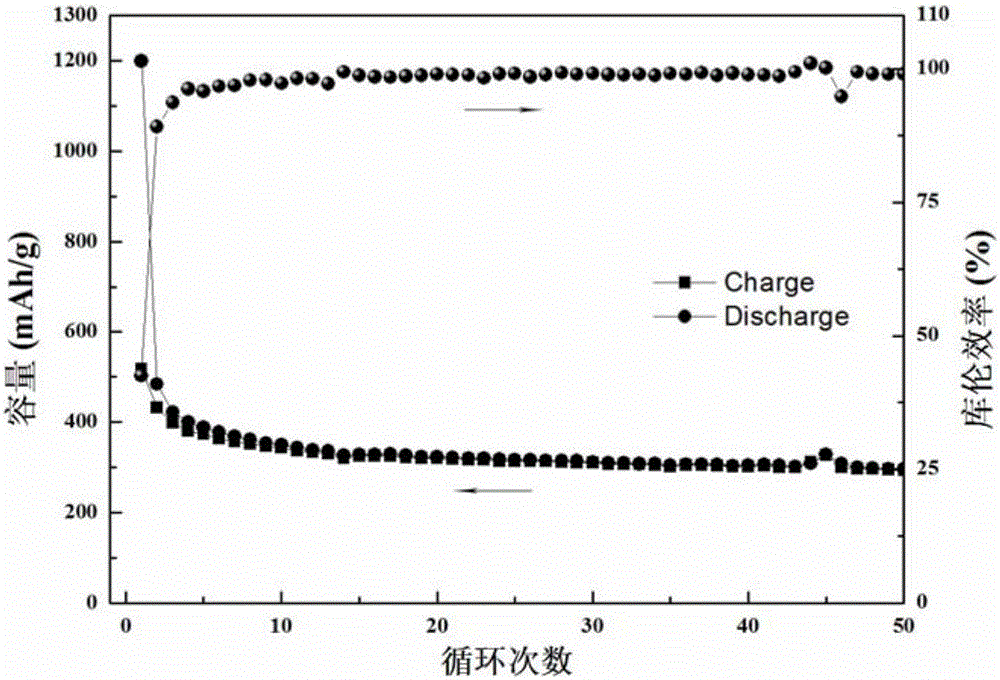
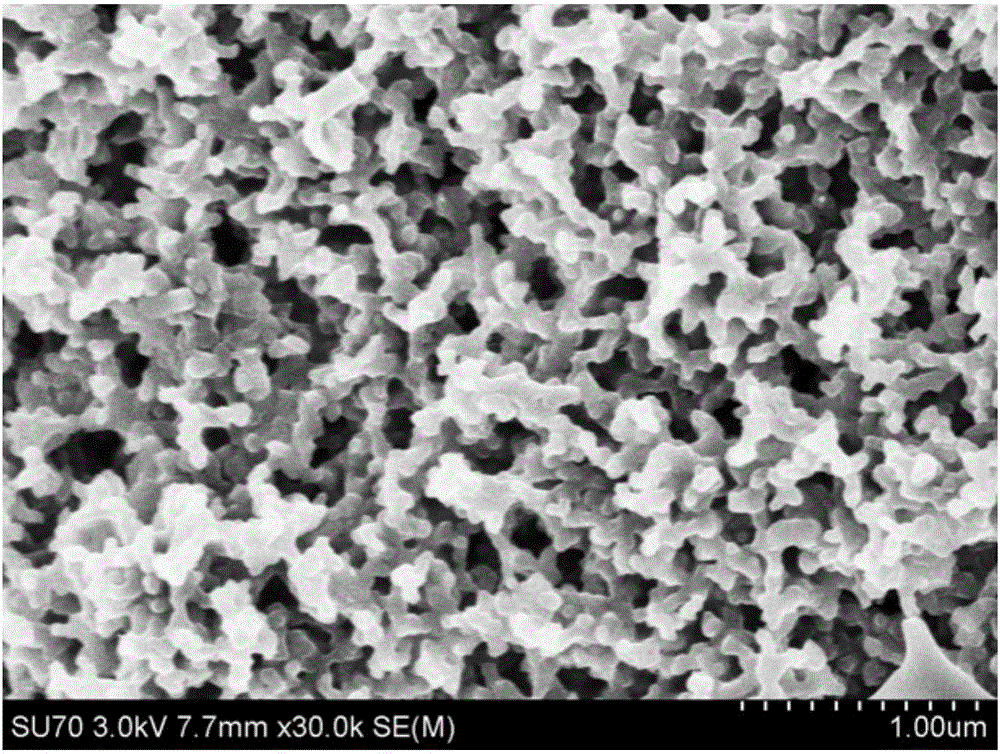
[0044] (5) Put the airgel obtained in (4) into a tube furnace, and carbonize it at 700°C for 3 hours to obtain nitrogen-doped carbon airgel. The SEM picture is as follows figure 2 shown. Such as figure 1 As shown, the nitrogen content of the nitro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com