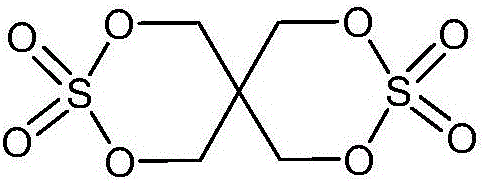
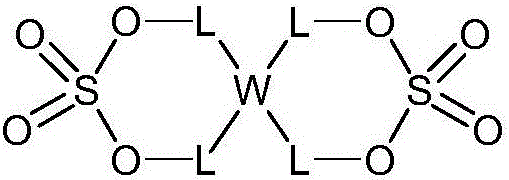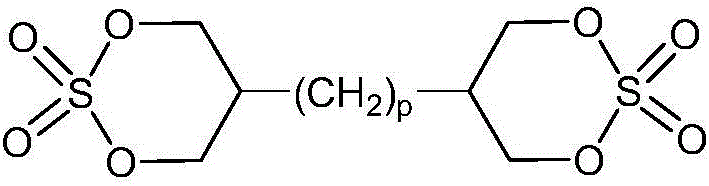Electrolyte for Lithium Secondary Battery and Lithium Secondary Battery Containing the Same
A technology for secondary batteries and electrolytes, applied in the field of electrolytes for lithium secondary batteries and lithium secondary batteries containing them, capable of solving problems such as inability to guarantee safety, problems with storage characteristics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0162] [Example 1] Synthesis of a compound represented by Chemical Formula 2
[0163]
[0164] 13.6 g of pentaerythritol, 100 ml of tetrahydrofuran, and 18 ml of thionyl chloride were sequentially injected into a 250 ml flask, and the mixture was refluxed and stirred. The hydrogen chloride gas formed was neutralized by passing it through aqueous sodium hydroxide solution. The crystals formed by stirring overnight were filtered and washed three times with 100 ml of diethyl ether. After placing the crystals in a 250ml flask, inject 104mg of ruthenium trichloride (RuCl 3 ) and 50ml acetonitrile. After using an ice bath to cool the reactant, slowly inject 140ml of 10% sodium hypochlorite aqueous solution into it and stir for 30 minutes, and then add 630mg of sodium sulfite (Na 2 SO 3 ) to terminate the reaction. Acetonitrile was removed by distillation under reduced pressure and the solid formed was filtered. The obtained solid was washed twice with 100 ml of water, and d...
Embodiment 2
[0166] [Example 2] Synthesis of compounds represented by Chemical Formula 5
[0167]
[0168] 2.4g mercury sulfate (HgSO 4 ) and 100g of tetrachloroethane were injected into the 1L flask in sequence, N 2 400 ml of 65% oleum was slowly injected thereinto over 2 hours under atmosphere. After the injection was terminated, stirring was performed for 8 hours while maintaining the temperature at 60°C. When the stirring was completed, the reactant was slowly poured into a 2 L beaker filled with 1 L of ice water, white crystals were formed by stirring the mixture for 1 hour and the crystals were filtered. The obtained crystals were washed four times with 500 ml of cold water, and dried in a vacuum oven to obtain the title compound (90 g).
[0169] 1 H-NMR (500MHz, DMSO) δ: 8.15 (s, 2H).
Embodiment 3
[0170] [Example 3] Synthesis of compounds represented by Chemical Formula 4-7
[0171]
[0172] 5.03g 1,5-cyclooctadiene, 50ml acetone and 58mg osmium tetroxide (OsO 4 ) into 100ml flasks in sequence, and cooled with an ice bath. 13.5 g of N-methylmorpholine N-oxide and 12.5 ml of water were poured thereinto, followed by stirring for 1 hour. When stirring was complete, the reaction temperature was raised to room temperature by removing the ice bath, and the mixture was stirred for 18 hours. The solid formed was filtered, washed with 20 ml of acetone, stirred with 3 ml of water and 30 ml of acetonitrile for 2 hours, then filtered. After the obtained solid was boiled with toluene to remove residual water, residual toluene was removed by vacuum drying to obtain 4.2 g of cyclooctane-1,2,5,6-tetraol (A-1).
[0173] Inject 4g of cyclooctane-1,2,5,6-tetraol (A-1) and 40ml of tetrahydrofuran into a 100ml flask in sequence, cool with an ice bath, slowly inject 5.9g of thionyl chlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com