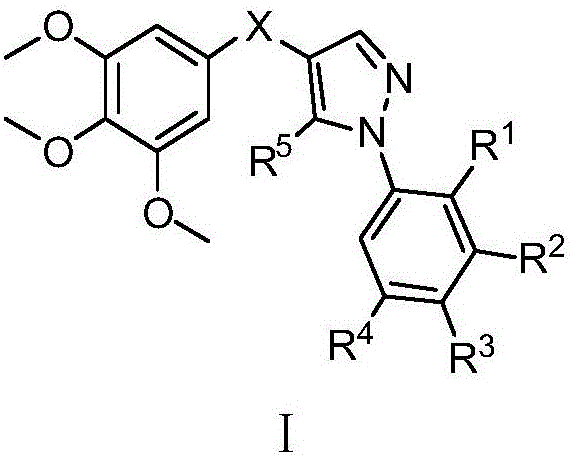
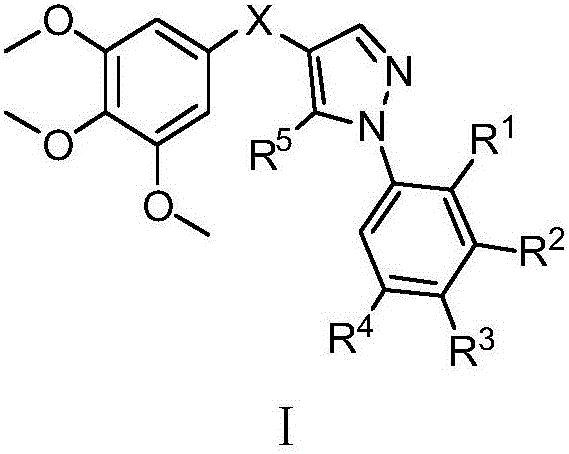
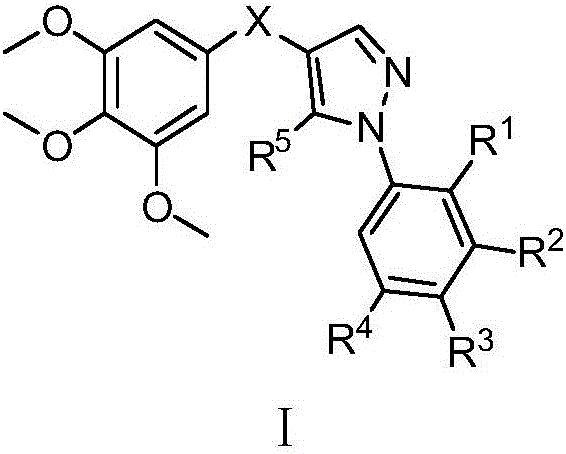
(1-aryl-1h-pyrazol-4-yl)(3,4,5-trimethoxyphenyl)ketone and ketoxime compounds and application thereof
A technology of trimethoxyphenyl and methylphenyl, which is applied in the field of medicine and can solve the problems that the research on anti-tumor activity has not been reported yet.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0181] Embodiment 1: Preparation of 3-anilino-2-(3,4,5-trimethoxybenzoyl)acrylonitrile
[0182] 3,4,5-trimethoxybenzaldehyde (10.0g, 50.97mmol), potassium hydroxide (8.56g, 152.91mmol), anhydrous copper chloride (0.087g, 0.51mmol), anhydrous acetonitrile (20.92g ,509.68mmol), mixed with 50mL of anhydrous N,N'-dimethylacetamide, and reacted for 12 hours under an oxygen atmosphere. 5. Precipitate a pale yellow solid, filter and dry to obtain the compound 3-oxo-3-(3,4,5-trimethoxyphenyl)propionitrile, which is directly used in the next step without purification. The compound 3-oxo-3-(3,4,5-trimethoxyphenyl)propionitrile (5.0g, 21.25mmol), N,N'-diphenylformamidine (4.17g, 21.25mmol) was added In the reaction bottle, add xylene to dissolve, and reflux reaction under nitrogen protection condition for 2 hours. The xylene was distilled off under reduced pressure, and the compound 3-phenylamino-2-(3,4,5-trimethoxybenzoyl)acrylonitrile could be obtained through separation and purifica...
Embodiment 2
[0183] Example 2: Preparation of (5-amino-1-phenyl-1H-pyrazol-4-yl)(3,4,5-trimethoxyphenyl)methanone (compound 1)
[0184] Add 3-anilino-2-(3,4,5-trimethoxybenzoyl)acrylonitrile (1.0g, 2.96mmol) and phenylhydrazine hydrochloride (0.43g, 2.96mmol) into the microwave reaction tube, Add an appropriate amount of ethanol to dissolve, add triethylamine (0.30g, 2.96mmol), and react in microwave at 120°C for 20 minutes. After the reaction is completed, the ethanol is evaporated under reduced pressure, and compound 1 can be obtained by separation and purification by column chromatography. Yield 86%. 1 H NMR (400MHz, CDCl 3 ): δ3.93(9H,s),6.09(2H,s),7.09(2H,s),7.44(1H,m),7.55(4H,m),7.85(1H,s)ppm; MS(ESI ):[M+H] + =354.1, [M+Na] + = 376.1.
Embodiment 3
[0185] Example 3: Preparation of (1-phenyl-1H-pyrazol-4-yl)(3,4,5-trimethoxyphenyl)methanone (compound 2)
[0186] Add compound 1 (0.50g, 1.42mmol) into an eggplant-shaped bottle, dissolve it with an appropriate amount of tetrahydrofuran, add isoamyl nitrite (0.33, 2.84mmol) dropwise, and react at room temperature for 2 hours. Compound 2 can be obtained by separation and purification by column chromatography. Yield 70%. 1 H NMR (400MHz, CDCl 3 ): δ3.92(6H,s),3.94(3H,s),7.17(2H,s),7.38(1H,t,J=7.4Hz),7.50(2H,dd,J=8.2,J=7.6 Hz), 7.75(2H,d,J=7.6Hz), 8.15(1H,s), 8.50(1H,s)ppm; MS(ESI):[M+H] + =339.1, [M+Na] + = 361.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com