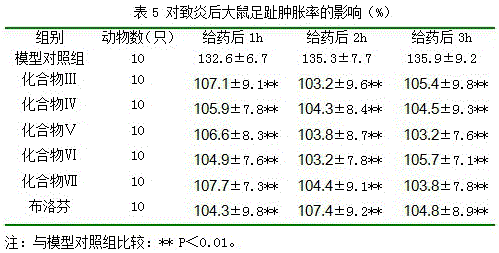
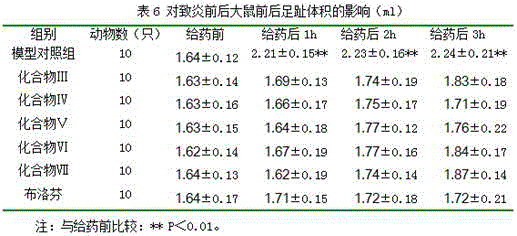
Anti-inflammatory compound
A compound, selected technology, applied in anti-inflammatory agents, drug combinations, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
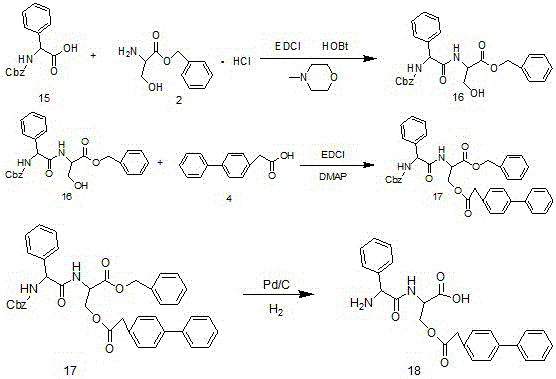
[0027] Embodiment 1: Preparation of series compound II
[0028] The synthetic route is shown in the following reaction formula:
[0029]
[0030] Under nitrogen atmosphere, compound 1 (10.5g, 0.05mol), compound 2 (11.6g, 0.05mol), HOBT (7.5g, 0.055mol) and N-methylmorpholine (16.5g, 0.016mol) were dissolved in two To methyl chloride (160ml), EDCI (10.6g, 0.055mol) was added in batches as a solid under ice-cooling conditions. After the dropwise addition, stir and react at 0°C for 30 minutes, remove the ice bath, and react at room temperature for 6 hours. TLC detects that the reaction is complete (DCM / MeOH=10 / 1), and the organic phase is sequentially washed with 1M dilute hydrochloric acid and saturated NaHCO 3 The aqueous solution, water and saturated saline were washed once respectively, dried over anhydrous sodium sulfate, and the solvent was evaporated to obtain 16.5 g of a white solid product (yield 85%).
[0031] Under nitrogen atmosphere, compound 3 (16.5g, 0.043mol)...
Embodiment 2
[0033] Embodiment 2: Preparation of series compound III
[0034] The synthetic route is shown in the following reaction formula:
[0035]
[0036]Under nitrogen atmosphere, compound 7 (11.2g, 0.05mol), compound 2 (11.6g, 0.05mol), HOBT (7.5g, 0.055mol) and N-methylmorpholine (16.5g, 0.016mol) were dissolved in di To methyl chloride (160ml), EDCI (10.6g, 0.055mol) was added in batches as a solid under ice-cooling conditions. After the dropwise addition, stir and react at 0°C for 30 minutes, remove the ice bath, and react at room temperature for 6 hours. TLC detects that the reaction is complete (DCM / MeOH=10 / 1), and the organic phase is sequentially washed with 1M dilute hydrochloric acid and saturated NaHCO 3 The aqueous solution, water and saturated brine were washed once, dried over anhydrous sodium sulfate, and the solvent was evaporated to obtain 18.0 g of the product (yield 90%).
[0037] Under a nitrogen atmosphere, compound 8 (17g, 0.043mol), compound 4 (9g, 0.043mo...
Embodiment 3
[0039] Embodiment 3: the preparation of series compound IV
[0040] The synthetic route is shown in the following reaction formula:
[0041]
[0042] Under nitrogen atmosphere, compound 11 (12.6g, 0.05mol), compound 2 (11.6g, 0.05mol), HOBT (7.5g, 0.055mol) and N-methylmorpholine (16.5g, 0.016mol) were dissolved in di To methyl chloride (160ml), EDCI (10.6g, 0.055mol) was added in batches as a solid under ice-cooling conditions. After the dropwise addition, stir and react at 0°C for 30 min, remove the ice bath, and react at room temperature for 6 h. TLC detects that the reaction is complete (DCM / MeOH=10 / 1), and the organic phase is washed with 1M dilute hydrochloric acid, saturated NaHCO3 aqueous solution, water and saturated Each was washed once with saline, dried over anhydrous sodium sulfate, and the solvent was evaporated to obtain 20 g of a white solid product (93% yield).
[0043] Under nitrogen atmosphere, compound 12 (18.4g, 0.043mol), compound 4 (9g, 0.043mol) an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com