Polyfuran compound and preparation method thereof
A compound and polyfuran technology, applied in the field of polyfuran compounds and their preparation, can solve the problems of limited oxygen oxidation system development, limited reaction applicability, difficult synthesis of reaction substrates, etc., and achieves easy availability of catalysts and excellent processability. , the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
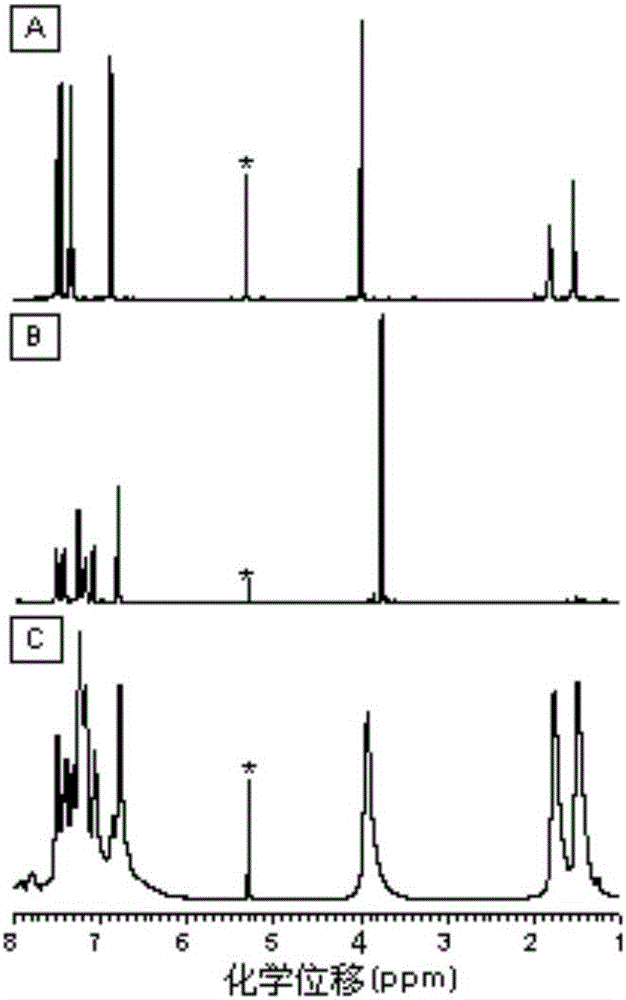
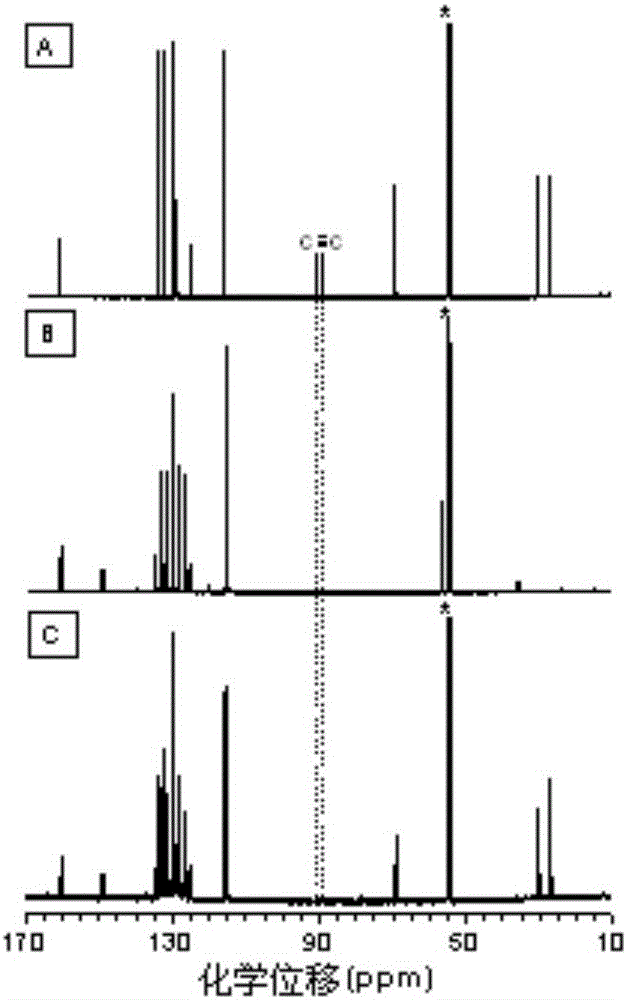
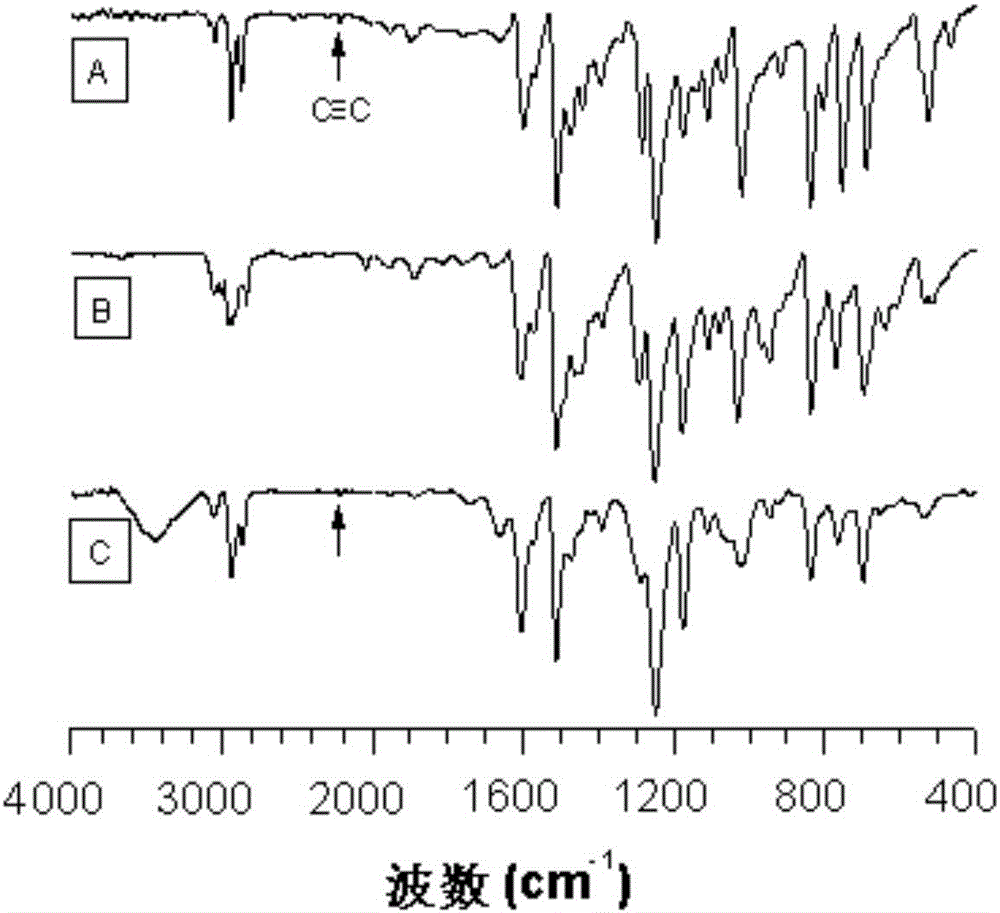
Image
Examples
Embodiment 1
[0047] A kind of polyfuran compound of the present embodiment, its structural formula is as shown in P1 (6):
[0048]
[0049] The above-mentioned polyfuran compound is prepared by the polymerization reaction of difunctional aryl internal alkyne monomer and oxygen, and the reaction equation is as formula (1):
[0050]
[0051] Wherein, the synthesis method of the monomer M1 can be synthesized according to the synthesis method of the applicant in the published literature (Polym. Chem., 2013, 4, 2841-2849.).
[0052] The specific preparation steps of the polyfuran compound described in the present embodiment are as follows:
[0053] In a dry Schlenk tube add M1 (94mg, 0.2mmol), Pd(OAc) 2 (20mol%), ZnCl 2 (60mol%), evacuated for 0.5 hours, plunged into a balloon full of oxygen, added N,N-dimethylacetamide (DMAc) and perfluorodecalin (C 10 f 18 ) each 0.5mL, reacted at 70°C for 8 hours, and then cooled to room temperature. After the reaction, the solution was passed thro...
Embodiment 2
[0059] A kind of polyfuran compound of the present embodiment, its structural formula is as shown in P1 (4):
[0060]
[0061] The above-mentioned polyfuran compound is prepared by the polymerization reaction of a difunctional aryl internal alkyne monomer and oxygen, and the reaction equation is as formula (3):
[0062]
[0063] Wherein, the synthesis method of the monomer M2 can be synthesized according to the synthesis method of the applicant in the published literature (Polym. Chem., 2013, 4, 2841-2849.).
[0064] The specific preparation steps of the polyfuran compound described in the present embodiment are as follows:
[0065] In a dry Schlenk tube was added M2 (88.5 mg, 0.2 mmol), Pd(OAc) 2 (20mol%), ZnCl 2 (60mol%), evacuated for 0.5 hours, plunged into a balloon full of oxygen, added N,N-dimethylacetamide (DMAc) and perfluorodecalin (C 10 f 18 ) each 0.5mL, reacted at 70°C for 8 hours, and then cooled to room temperature. After the reaction, the solution was...
Embodiment 3
[0069] A kind of polyfuran compound of the present embodiment, its structural formula is as shown in P1 (8):
[0070]
[0071] The above-mentioned polyfuran compound is prepared by the polymerization reaction of difunctional aryl internal alkyne monomer and oxygen, and the reaction equation is as formula (4):
[0072]
[0073] Wherein, the synthesis method of the monomer M3 can be synthesized according to the synthesis method of the applicant in the published literature (Polym. Chem., 2013, 4, 2841-2849.).
[0074] The specific preparation steps of the polyfuran compound described in the present embodiment are as follows:
[0075] In a dry Schlenk tube was added M3 (99.7 mg, 0.2 mmol), Pd(OAc) 2 (20mol%), ZnCl 2 (60mol%), evacuated for 0.5 hours, plunged into a balloon full of oxygen, added N,N-dimethylacetamide (DMAc) and perfluorodecalin (C 10 f 18 ) each 0.5mL, reacted at 70°C for 8 hours, and then cooled to room temperature. After the reaction, the solution was p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com